FDA Approves Keytruda for Biliary Cancer, Rivals AstraZeneca’s Imfinzi
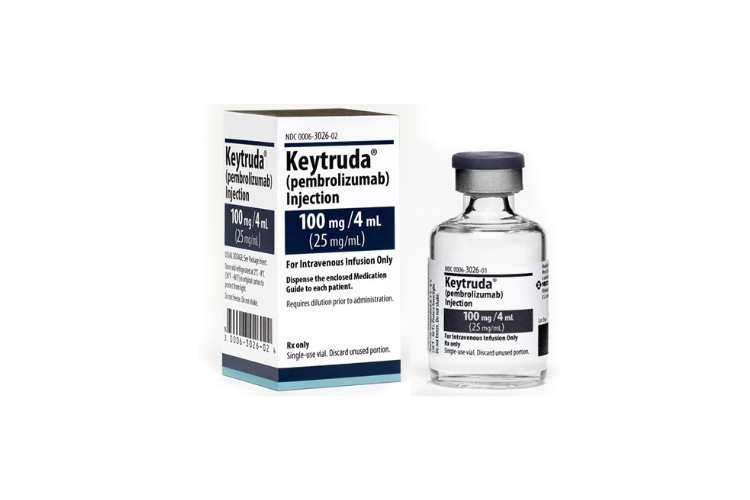
Merck’s Keytruda has received FDA approval to treat locally advanced unresectable or metastatic biliary tract cancer, alongside gemcitabine and cisplatin chemotherapy, marking its entry into this challenging cancer space. This approval signifies Keytruda’s sixth FDA nod for gastrointestinal cancer treatment. The FDA’s decision was based on data from Merck’s Phase 3 KEYNOTE-966 trial, demonstrating that … Read more