Roche

Roche Unveils Interim TIGIT Overall Survival Data Following Unintended Release, Triggering Surge in Stocks
An unexpected revelation has provided the world with a sneak peek into Roche’s highly anticipated TIGIT data. Roche, which had ...
Eli Lilly Sets Example for Roche with Positive Retevmo Results in Thyroid Cancer, Highlighting Feasibility of Trial
Just a few weeks ago, Roche had expressed doubts about the feasibility of a Phase 3 study involving its RET ...
Resilient Triumph: Roche and Exelixis’ Tecentriq-Cabometyx Combination Shows Promise in Advanced Prostate Cancer After Past Challenges
After facing setbacks in clinical trials, the combination of Roche’s Tecentriq and Exelixis’ Cabometyx has achieved a breakthrough in treating ...

Seagen’s Tukysa Demonstrates Trial Victory in Breast Cancer, Amplifying Roche’s Kadcyla and Significance in Light of Pfizer’s $43B Acquisition
In a strategic move, Pfizer has announced a hefty investment of $43 billion to acquire Seagen, with a keen focus ...

FDA Grants Approval to Ipsen’s Sohonos Capsules, the First-Ever Treatment for Individuals with Fibrodysplasia Ossificans Progressiva
In 2019, Ipsen made a significant investment of $1 billion to acquire Clementia Pharmaceuticals and its rare disease drug, palovarotene. ...

Genentech was fined $158K by the EPA for improper management of hazardous waste at a site in San Francisco
After reaching an agreement with the US Environmental Protection Agency (EPA) to address environmental violations at a former facility, Genentech ...
Regeneron’s High-Dose Eylea Demonstrates Persistence as FDA Decision Looms
While awaiting an FDA decision regarding a higher-dose version of Eylea (aflibercept), Regeneron has consistently emphasized the durability of their ...

Daiichi Sankyo to Dominate Antibody-Drug Conjugates Market by 2029
Daiichi has successfully established ADCs (Antibody-Drug Conjugates) as a significant therapeutic approach, overcoming previous years of underwhelming results. Even though ...

Roche Halts Development of Tenecteplase for Stroke
Roche announced today that it has discontinued the development of its thrombolytic drug tenecteplase for acute ischemic stroke due to ...

Roche & Alnylam partner: Hypertension RNAi Therapy – Zilebesiran
Source – Roche Roche has recently unveiled an exciting collaboration with Alnylam to jointly develop and commercialize zilebesiran, a promising ...

Roche’s Potential $7billion Acquisition: Exploring the Purchase of Roivant’s Promising Drug
According to media reports, Roche is said to be in the final stages of negotiations with Roivant for a potential ...
Promising Phase III Results: OCREVUS (Ocrelizumab) Subcutaneous Injection Offers Convenient and Efficient Treatment for Multiple Sclerosis
Source – Roche On 13 July Roche announced positive results from the Phase III OCARINA II trial, which investigated the ...

Roche’s Columvi (glofitamab) for patients with R/R diffuse large B-cell lymphoma has received approval from the European Commission
Source – Roche On 11 July 2023 Roche announced that the European Commission (EC) has given conditional approval for Columvi ...
Roche withdraws Gavreto approval, citing study impracticality
Roche’s cancer drug Gavreto was withdrawn for the treatment of advanced RET-mutant medullary thyroid cancer, following discussions with the FDA. ...
Roche’s Columvi and AbbVie’s Epkinly: The Battle for Breakthroughs in Large b Cell Lymphoma Treatment
Roche’s Columvi (glofitamab) receives FDA accelerated approval for treatment of Large b Cell Lymphoma, sparking a rivalry with AbbVie and ...

Glofitamab-gxbm receives FDA approval for relapsing or resistant DLBCL
Source – Roche Columvi (Glofitamab-gxbm) has been given expedited clearance by the FDA for the treatment of adult patients with ...
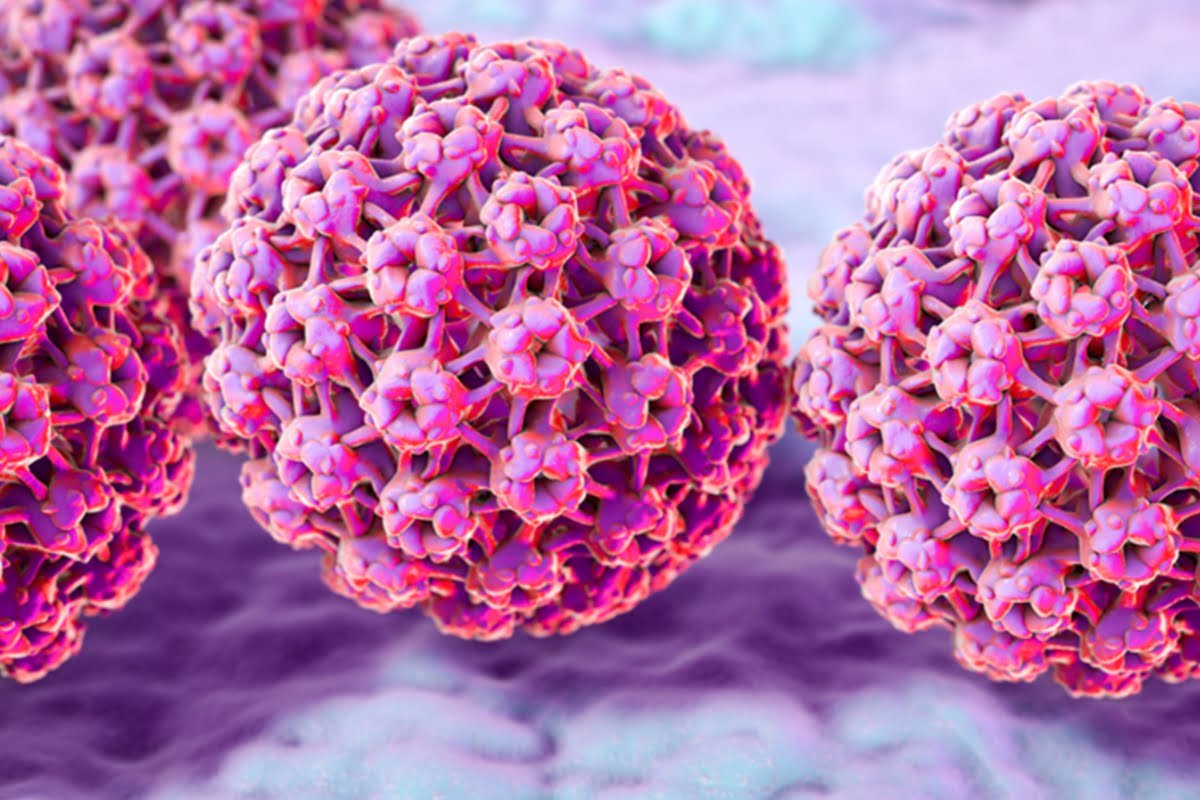
In order to provide access to cervical cancer screening tools in low- and lower-middle income countries, Roche received WHO prequalification for the Cobas HPV test
Source – Roche On June 13, 2023, the World Health Organisation (WHO) prequalified the Cobas Human Papillomavirus (HPV) test for ...

Roche’s HER2 Powerhouse: Leading the Way in Breast Cancer Treatment
Breast cancer treatment was primarily based on the tumor’s location for most of the previous century, resulting in varying outcomes. ...









