FDA

The US FDA has approved the use of Jardiance (empagliflozin) to treat type II diabetes in children and adolescents aged 10 and older
Source – Eli Lilly On June 21, 2023, Boehringer Ingelheim and Eli Lilly and Company announced that the US Food ...
A monovalent COVID booster is advised by the FDA advisory group
The FDA advisory panel recently reached a unanimous conclusion regarding COVID-19 vaccinations for the upcoming autumn. They recommended a transition ...
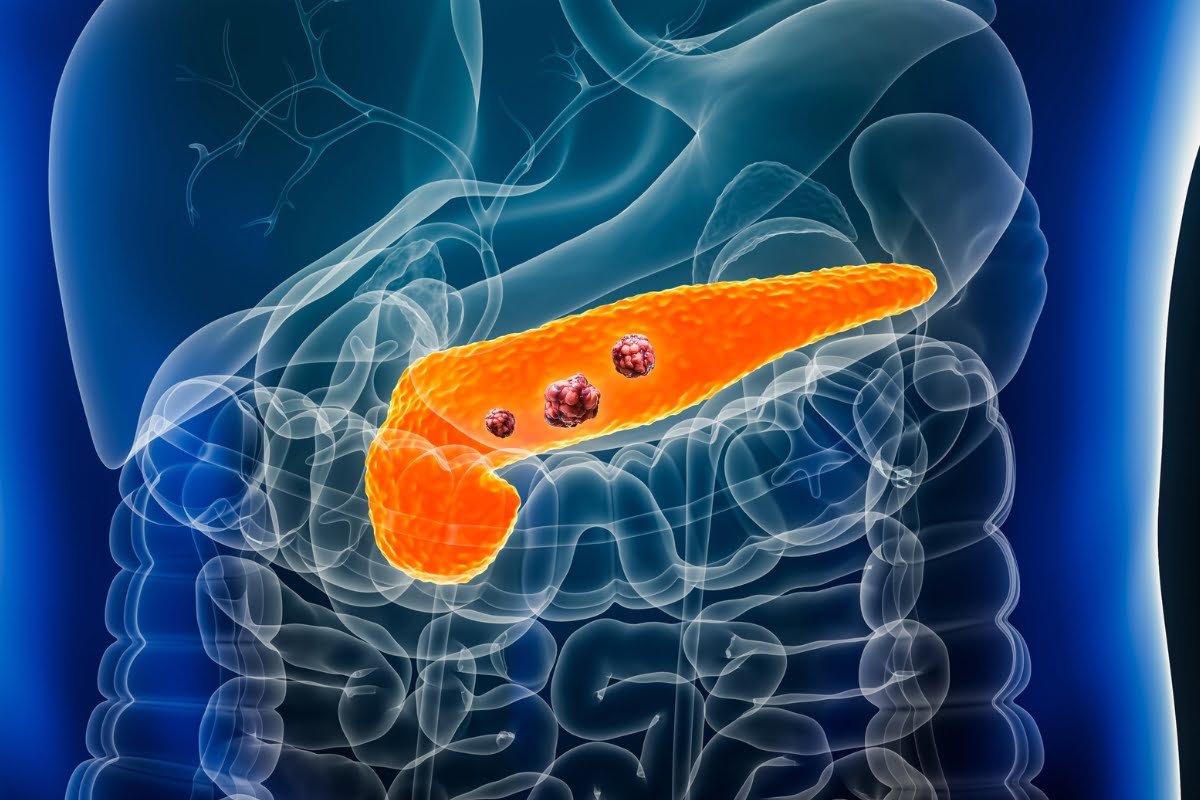
The FDA supports a biomarker for aggressive fibrotic tumors
Source: Nordic Bio The FDA has granted its backing to a blood test that may be able to determine if ...
FDA committee to talk about choosing the right strain for the subsequent round of COVID-19 boosts
Source – FDA The FDA’s Advisory Committee on Vaccines and Related Biological Products will meet on 15 June to discuss ...

FDA Prolongates PDUFA Nirogacestat’s NDA for desmoid tumors is three months
Source – SpringWorks According to SpringWorks Therapeutics on June 7, 2023, the FDA has extended the Prescription Drug User Fee ...

Companion Diagnostic for Encorafenib + Cetuximab in BRAF V600E alteration in mCRC Receives FDA Approval
Source – Foundation Medicine In order to identify patients with metastatic colorectal cancer (mCRC) who could be candidates to receive ...

Acadia plans a Phase III study for a Prader-Willi syndrome candidate following an FDA meeting
Source – Acadia Pharmaceuticals ACP-101, also known as intranasal carbetocin, has been chosen by Acadia to move forward into a ...

Huma Received FDA Class II clearance for the SaMD platform
Source – HUMA With the FDA’s Class II clearance of its Software as a Medical Device (SaMD) disease management platform, ...

Valneva Preparing for the FDA’s decision on the chikungunya vaccine
Just a few months away from the FDA making a judgment on Valneva’s marketing application for the chikungunya vaccine VLA1553, ...

FDA authorizes the first medication for functional constipation in children
Source- FDA Pediatric patients aged 6 to 17 may use Linzess (linaclotide) pills to treat functional constipation. The first therapy ...

Futura receives FDA approval for OTC treatment for erectile dysfunction
Source- Futura Medical The first business in the US to provide a topical erectile dysfunction medication to the market without ...
SAR’579/IPH6101 Granted FDA Fast Track Designation for Hematological Malignancies
Source: Innate Pharma On June 6, 2023, the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation to ...
FDA Advisory Committee Unanimously Recommends Nirsevimab for Preventing RSV Lower Respiratory Tract Disease in Infants
Source: AstraZeneca The FDA’s Antimicrobial Drugs Advisory Committee (AMDAC) has voted unanimously, with a 21 to 0 decision, affirming the ...
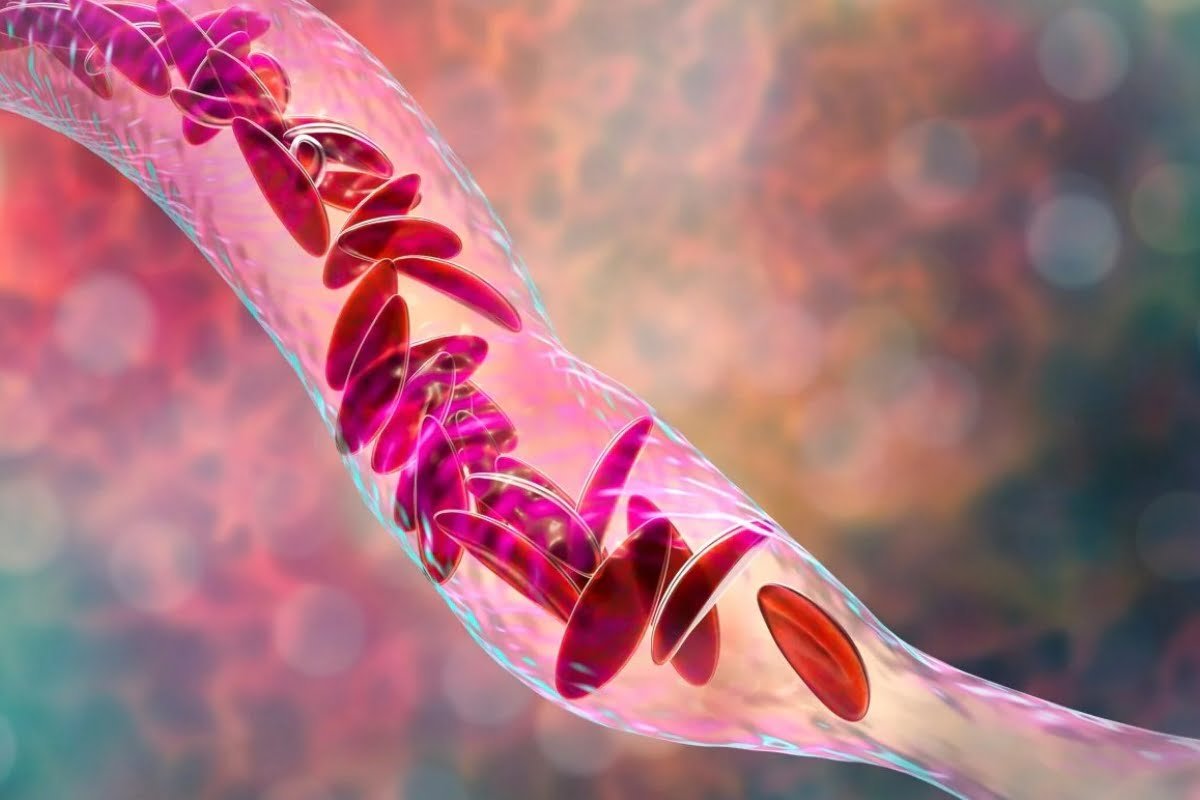
FDA Sets December Deadline for Vertex and CRISPR’s Sickle Cell Therapy
The US Food and Drug Administration (FDA) has initiated a priority review of exagamglogene autotemcel (exa-cel), a therapy developed by ...
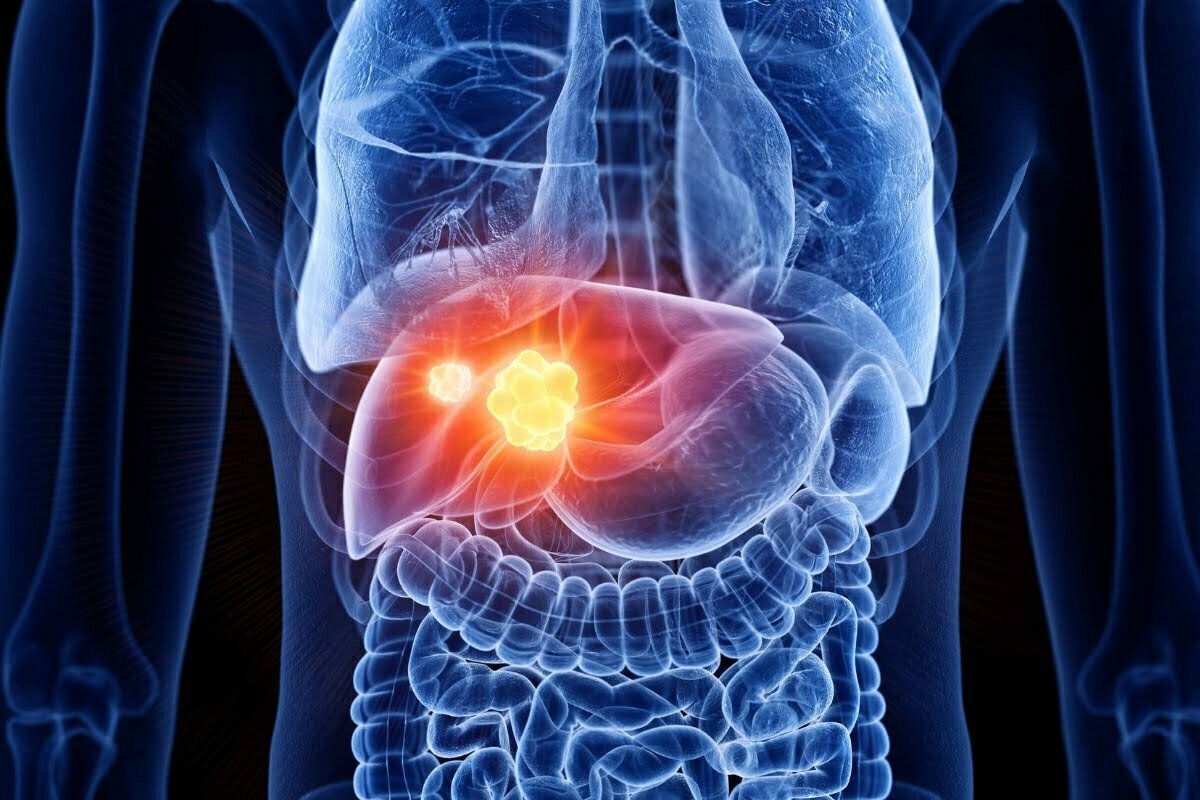
FDA accepts Merck’s application for Keytruda plus chemo in liver cancer
The FDA has granted acceptance to Merck’s application for the popular drug Keytruda, in combination with standard-of-care chemotherapy (gemcitabine plus ...
Johnson & Johnson (J&J) Moves Closer to FDA Approval for CAR-T Therapy
The company has filed for approval to use Carvykti earlier in the treatment pathway, including for patients experiencing their first ...

FDA sets the stage for adcomm on Sanofi, AZ’s RSV prospect later this week
GSK and Pfizer have plans to introduce their adult respiratory syncytial virus (RSV) vaccines later this year, while Sanofi and ...

ALLERGAN’S LATEST SKIN-SMOOTHING PRODUCT HAS RECEIVED FDA APPROVAL, FEATURING A UNIQUE DELIVERY MECHANISM
Allergan has recently obtained FDA approval for its groundbreaking skincare product, Skinvive, which is part of the Juvederm dermal filler ...
EPCORITAMAB RECEIVES FDA APPROVAL FOR RELAPSED/REFRACTORY DLBCL
The FDA has granted approval to epcoritamab-bysp (Epkinly), the first T-cell–engaging bispecific antibody, for the treatment of relapsed/refractory diffuse large ...
![THE U.S. FOOD AND DRUG ADMINISTRATION (FDA) HAS ISSUED A COMPLETE RESPONSE LETTER CONCERNING [VIC-]TRASTUZUMAB DUOCARMAZINE, A PRODUCT DEVELOPED BY BYONDIS.](https://pharmtales.com/wp-content/uploads/2023/06/FDA-Issues-Response-Letter-on-Byondis-Trastuzumab-Duocarmazine.jpg)
FDA Issues Response Letter on Byondis’ Trastuzumab Duocarmazine
Byondis B.V., a Dutch clinical-stage biopharmaceutical company specializing in precision medicines, has announced today that the U.S. Food and Drug ...