Source – Johnson & Johnson
On July 4, 2023, Janssen, a subsidiary of Johnson & Johnson announced positive initial results from the Phase IIb FRONTIER 1 trial, which evaluated the effectiveness of JNJ-2113, a new oral interleukin-23 receptor antagonist peptide, in adults with moderate-to-severe plaque psoriasis.
JNJ-2113 is an oral peptide that functions as an IL-23 receptor antagonist, specifically binding to the IL-23 receptor. The IL-23/IL-23R signaling pathway plays a crucial role in the development of plaque psoriasis and other immune-mediated inflammatory diseases. By selectively inhibiting IL-23 signaling and subsequent inflammatory cytokine production, JNJ-2113 presents a promising treatment option for patients with plaque psoriasis.
“The development of a novel oral therapy that specifically targets IL-23R could potentially change the treatment paradigm for patients living with moderate-to-severe plaque psoriasis. Until now, advanced psoriasis treatments have been largely limited to injectable biologics. An oral therapy that can uniquely inhibit the IL-23 pathway by directly targeting the IL-23 receptor could help address the needs and preferences of patients, and may offer greater freedom, with the aim of driving greater adoption of advanced treatment.”
– Dr Lloyd Miller, vice president and immunodermatology disease area stronghold leader at Janssen Research & Development
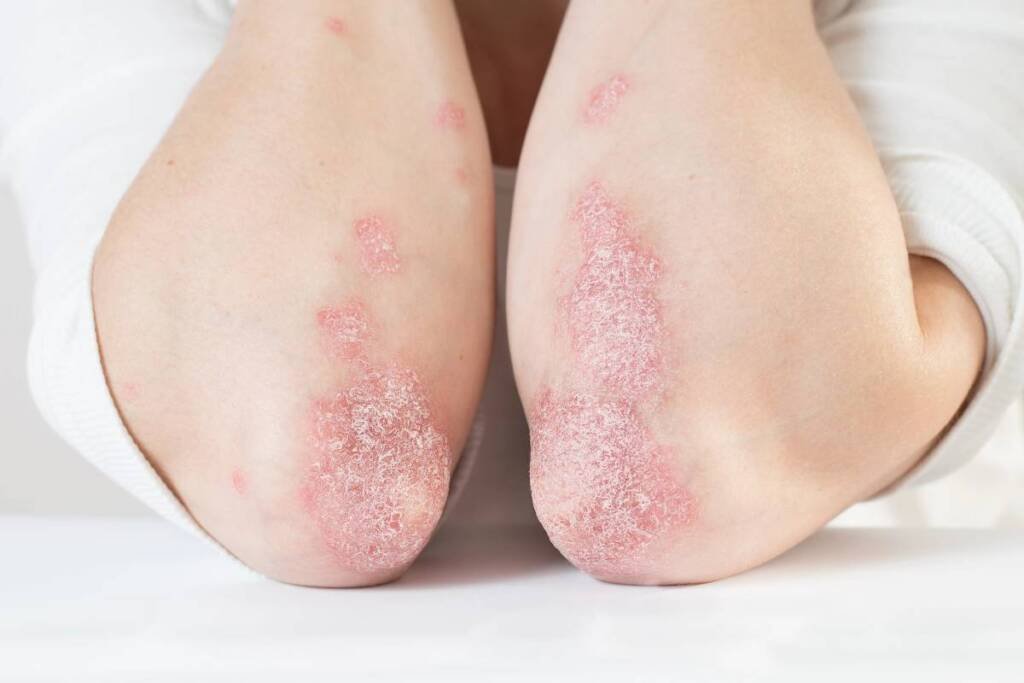
Plaque psoriasis is an immune-mediated condition estimated to impact over 125 million people globally. The disease leads to excessive production of skin cells, resulting in inflamed, scaly plaques that can cause itching or pain, significantly affecting patients’ quality of life, emotional well-being, and relationships.
In the randomized, double-blind, placebo-controlled trial, researchers assessed the efficacy and safety of JNJ-2113 in patients who had previously failed at least one systemic therapy.
Results disclosed by Janssen demonstrate that the trial successfully achieved its primary objective by showing that a greater proportion of patients treated with JNJ-2113 experienced significant improvement in their skin condition, as measured by the Psoriasis Area and Severity Index (PASI), compared to the placebo group at week 16. Across different dosage groups (25mg daily and 100mg twice daily) of JNJ-2113, 37.2% to 78.6% of patients achieved PASI 75, while only 9.3% of the placebo group achieved the same result.
Additionally, JNJ-2113 also met its secondary efficacy endpoints. The percentage of patients achieving PASI 90 ranged from 25.6% (25mg daily dose) to 59.5% (100mg twice-daily dose) in the JNJ-2113 groups, compared to only 2.3% in the placebo group. Moreover, JNJ-2113 groups exhibited significant improvement in PASI 100 compared to placebo, with results ranging from 9.8% to 40.5%, while the placebo group did not show similar improvements.
“Patients are looking for more flexible and convenient treatment options to manage the signs and symptoms of psoriasis, and these positive early results for JNJ-2113 are encouraging. The majority of people living with moderate-to-severe plaque psoriasis are eligible for but are still not receiving advanced therapies. For many patients, a pill is preferable to an injection.”
– Robert Bissonnette, M.D., FRCPC, Chief Executive Officer and Medical Director, Innovaderm, Montreal, Canada, and Lead Investigator of the FRONTIER clinical trial
Based on the positive findings from the FRONTIER 1 trial, Janssen intends to move forward with Phase III development of JNJ-2113 for the treatment of moderate-to-severe plaque psoriasis. Additionally, the company plans to initiate a Phase IIb clinical trial for adults with ulcerative colitis.