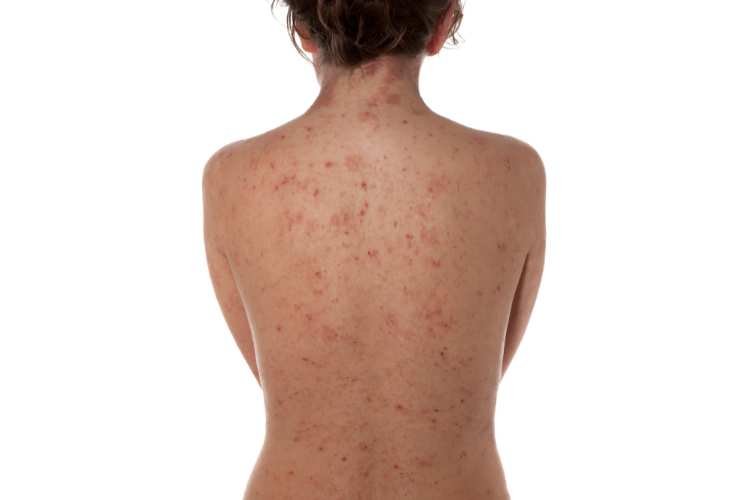
Evommune, a California-based biotech company, has made the strategic decision to discontinue its sole clinical-stage asset, EVO101, following a thorough evaluation of data from a phase 2 trial targeting atopic dermatitis in adults with mild to moderate severity.
“We executed a robust and well-controlled phase 2a study of EVO101 designed to show significant improvement over standard of care, and this allowed us to make an efficient decision based on the data. “We are thrilled to enter into this collaboration with Maruho, a globally respected organization that has developed and commercialized pharmaceuticals in Japan for over 100 years. We believe that this is the start of a long term, multifaceted relationship between Evommune and Maruho, with the potential to accelerate development and access to EVO756 for patients in Japan and around the world.”
– Luis Peña, President, and Chief Executive Officer
Also Read: Almirall Uses Famous Paintings To Raise Awareness Of Atopic Dermatitis
The interleukin 1 receptor-associated kinase 4 (IRAK4) inhibitor, EVO101, has garnered significant interest in the pharmaceutical industry, given the continued exploration of IRAK4 as a therapeutic target. Notably, Gilead Sciences made a substantial upfront payment of $20 million in March to license an IRAK4 degrader from Nurix, shortly after abandoning two out of three indications for its IRAK4 inhibitor, GS-5718.
With the discontinuation of EVO101, Evommune will now shift its focus and resources toward advancing its pipeline of systemically administered product candidates, which are expected to reach multiple milestones over the coming years.
One promising candidate in this pipeline is EVO756, an MRGPRX2 antagonist currently under development for urticaria and interstitial cystitis. Evommune has expressed confidence in EVO756, highlighting its potential as a groundbreaking oral treatment for various mast cell-mediated diseases and conditions involving sensory neuron-mediated cutaneous itch.
Also Read: Promising Phase IIb Results In Atopic Dermatitis: Amlitelimab As A Groundbreaking Treatment
In a significant development, Maruho has recognized the potential of EVO756, securing the rights to develop and commercialize this asset in Japan. The agreement involves a total of $60 million in combined upfront and potential milestone payments, underscoring the value and promise that EVO756 holds in the eyes of industry partners.
As Evommune sets its sights on launching EVO756 into clinical trials in early 2024, this strategic shift in focus signifies the company’s commitment to advancing innovative therapies for a range of challenging medical conditions.