Pharma
Stay updated with the latest happenings in the pharmaceutical industry. Get insights into drug development, innovations, regulatory updates, and emerging trends. Discover the advancements shaping the future of healthcare. Explore the world of pharma with our comprehensive coverage at Pharmtales.com.

Eisai has been targeted by a ransomware attack and has initiated an investigation into potential data breaches
Eisai, a pharmaceutical company, has become the latest victim of a ransomware attack. Following the cyberattack on Saturday night, the ...
ASCO 2023: Zanidatamab – A New Hope for HER2-Amplified Biliary Tract Cancers
ABSTRACT NUMBER – 4008 A subset of biliary tract cancer cases presents either with overexpression or amplification of the HER2 ...
ASCO 2023: Elicio Therapeutics Unveils Promising Interim Results from AMPLIFY-201 Phase I Trial of Cutting-Edge Cancer Immunotherapy
ABSTRACT NUMBER – 2528 ELI-002 represents a new therapeutic vaccine called AMP that specifically targets KRAS-driven cancers. KRAS mutations are ...
Sotorasib vs Docetaxel in KRAS G12C-Mutated NSCLC: ASCO 2023 Highlights
ABSTRACT NUMBER – LBA9016 Amgen has made a courageous endeavor in cancer research over the last four decades by creating ...
ASCO 2023: ELAHERE – A New Hope for Platinum-Resistant Ovarian Cancer Patients
ABSTRACT NUMBER – LBA5507 The MIRASOL trial, presented at ASCO on June 4, demonstrated a significant improvement in the primary ...

ASCO 2023: How Linvoseltamab Can Treat Pretreated Myeloma Patients
ABSTRACT NUMBER – 8006 Linvoseltamab, an investigational bispecific antibody known as BCMAxCD3, shows promising potential in connecting B-cell maturation antigen ...
ASCO 2023: Patients with HER2-Positive MCRC Benefit from Lower Dose of Trastuzumab Deruxtecan
ABSTRACT NUMBER – 3501 The Phase II DESTINY-CRC02 study, presented at the 2023 ASCO Annual Meeting, reported that administering ENHERTU ...
ASCO 2023: Promising Results from Sleeping Beauty TCR-T Cells
ABSTRACT NUMBER – 2547 Alaunos’ Clinical TCR Library focuses on targeting the most common mutations found in KRAS, TP53, and ...
ASCO 2023:Kelun Pharma’s SKB264 shows early signs of potential in NSCLC patients
ABSTRACT NUMBER – 9114 SKB264 is an innovative anti-TROP2 ADC that specifically targets a poor prognosis marker found in 70% ...

EpicentRx Provides Latest Updates on Oncology Clinical Development Programs at 2023 ASCO Annual Meeting
During the 2023 American Society of Clinical Oncology (ASCO) meeting, EpicentRx, Inc., a clinical-stage biopharmaceutical company, provided updates on the ...

ASCO 2023: BREYANZI Shines as First CAR T Therapy with Deep and Lasting Impact in Chronic Lymphocytic Leukemia
The initial findings from the important TRANSCEND CLL 004 study, which is evaluating the use of BREYANZI (lisocabtagene maraleucel) in ...
Pfizer’s RSV Vaccine ABRYSVO: A Breakthrough for the Industry and RSV Prevention
Pfizer’s RSV Vaccine: In a groundbreaking development, Pfizer’s FDA approval for its RSV vaccine, ABRYSVO, has not only positioned the ...
Pfizer’s Phase III Trials Bring Hope for Multidrug-Resistant Infections with Limited Treatment Options
On June 1, 2023, Pfizer announced encouraging outcomes from its Phase III program that involved the REVISIT and ASSEMBLE trials. ...
FDA Grants Approval to ABRYSVO, Pfizer’s Vaccine for Preventing Respiratory Syncytial Virus (RSV) in Elderly Individuals
The FDA, on May 31, 2023, granted approval to ABRYSVO (Respiratory Syncytial Virus Vaccine), Pfizer’s bivalent RSV prefusion F (RSVpreF) ...
OraPharma, a division of Bausch, teams up with Alex Rodriguez to tackle gum disease and aim for a victorious outcome
Bausch Health’s oral health division OraPharma is all set to launch a brand new gum disease awareness campaign “Cover Your ...
Lonza Bolsters Antibody-Drug Conjugates Portfolio with Synaffix Acquisition
Lonza, a global manufacturing company acquired Synaffix B.V., a biotechnology company specializing in the advancement of ADCs (antibody-drug conjugates) through ...
BRIUMVI Receives Green Light from European Commission as Treatment for Relapsing Multiple Sclerosis
The European Commission (EC) gave the green light for the use of TG Therapeutics‘ BRIUMVI (ublituximab-xiiy) to treat relapsing forms ...
Advancing Precision Oncology: Targeting KRAS Mutations with LUMAKRAS and KRAZATI
KRAS is a well-known oncogene that is highly prone to mutations in various cancers, including PDAC, NSCLC, and CRC. These ...
Unlocking the Future of Healthcare: The Rising Power of Gene Therapy
Gene therapy, a promising field at the intersection of genetics and medicine, is revolutionizing the way we approach disease treatment. ...

KRAS Mutations: Challenges and Breakthroughs in Cancer Treatment
Introduction Cancer remains a major global health concern, with various types of disease affecting millions of lives each year. One ...

Roche’s HER2 Powerhouse: Leading the Way in Breast Cancer Treatment
Breast cancer treatment was primarily based on the tumor’s location for most of the previous century, resulting in varying outcomes. ...

EU Regulators Call for Withdrawal of Authorization for Novartis’ Sickle Cell Drug Adakveo Following Phase 3 Setback
After the phase 3 trial of Novartis’ sickle cell disease drug, Adakveo, yielded disappointing results, regulators in Europe have revisited ...

Opdivo Receives Positive CHMP Opinion for Neoadjuvant Treatment of High-Risk NSCLC
On May 26, 2023, Bristol Myers Squibb announced that the European Medicines Agency’s Committee for Medicinal Products for Human Use ...
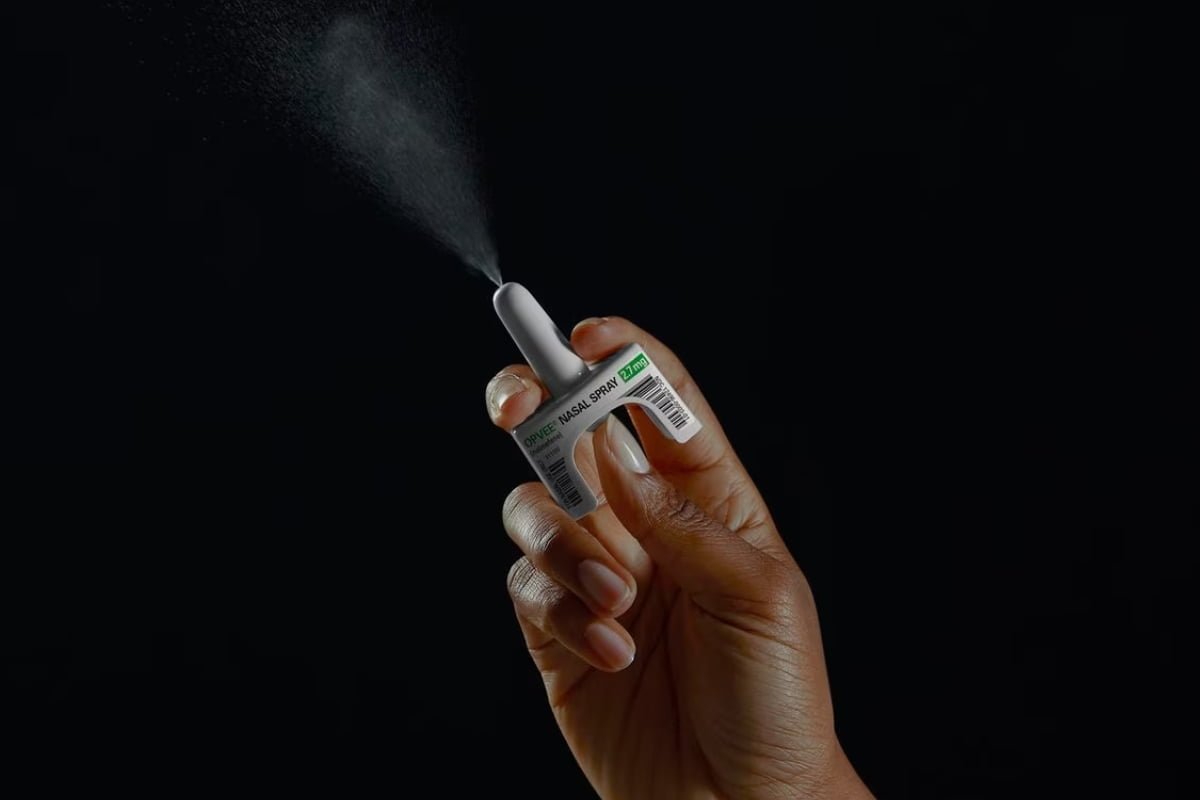
Indivior Receives FDA Approval for OPVEE (nalmefene)
Indivior’s OPVEE® (nalmefene) nasal spray has received FDA approval for the emergency treatment of opioid overdose in adults and pediatric ...
Epkinly: FDA-Approved Bispecific Antibody for DLBCL
On May 19, 2023, AbbVie announced that the U.S. Food and Drug Administration (FDA) granted approval to EPKINLYTM (epcoritamab-bysp) as ...

LEXICON ANNOUNCES FDA APPROVAL OF INPEFA (SOTAGLIFLOZIN) FOR TREATMENT OF HEART FAILURE
Lexicon Pharmaceuticals on May 26, 2023 announced that the US Food and Drug Administration (FDA) has approved INPEFA™ (sotagliflozin), a once-daily ...