Latest News
Get real-time updates on the latest news and developments in the pharmaceutical world. Stay informed about new drug launches, research findings, regulatory changes, and industry updates. Explore a wide range of topics and stay connected with the rapidly evolving world of pharma.

FDA sets the stage for adcomm on Sanofi, AZ’s RSV prospect later this week
GSK and Pfizer have plans to introduce their adult respiratory syncytial virus (RSV) vaccines later this year, while Sanofi and ...

Eisai has been targeted by a ransomware attack and has initiated an investigation into potential data breaches
Eisai, a pharmaceutical company, has become the latest victim of a ransomware attack. Following the cyberattack on Saturday night, the ...
Pfizer’s Phase III Trials Bring Hope for Multidrug-Resistant Infections with Limited Treatment Options
On June 1, 2023, Pfizer announced encouraging outcomes from its Phase III program that involved the REVISIT and ASSEMBLE trials. ...
FDA Grants Approval to ABRYSVO, Pfizer’s Vaccine for Preventing Respiratory Syncytial Virus (RSV) in Elderly Individuals
The FDA, on May 31, 2023, granted approval to ABRYSVO (Respiratory Syncytial Virus Vaccine), Pfizer’s bivalent RSV prefusion F (RSVpreF) ...
OraPharma, a division of Bausch, teams up with Alex Rodriguez to tackle gum disease and aim for a victorious outcome
Bausch Health’s oral health division OraPharma is all set to launch a brand new gum disease awareness campaign “Cover Your ...
Lonza Bolsters Antibody-Drug Conjugates Portfolio with Synaffix Acquisition
Lonza, a global manufacturing company acquired Synaffix B.V., a biotechnology company specializing in the advancement of ADCs (antibody-drug conjugates) through ...
BRIUMVI Receives Green Light from European Commission as Treatment for Relapsing Multiple Sclerosis
The European Commission (EC) gave the green light for the use of TG Therapeutics‘ BRIUMVI (ublituximab-xiiy) to treat relapsing forms ...

EU Regulators Call for Withdrawal of Authorization for Novartis’ Sickle Cell Drug Adakveo Following Phase 3 Setback
After the phase 3 trial of Novartis’ sickle cell disease drug, Adakveo, yielded disappointing results, regulators in Europe have revisited ...

Opdivo Receives Positive CHMP Opinion for Neoadjuvant Treatment of High-Risk NSCLC
On May 26, 2023, Bristol Myers Squibb announced that the European Medicines Agency’s Committee for Medicinal Products for Human Use ...
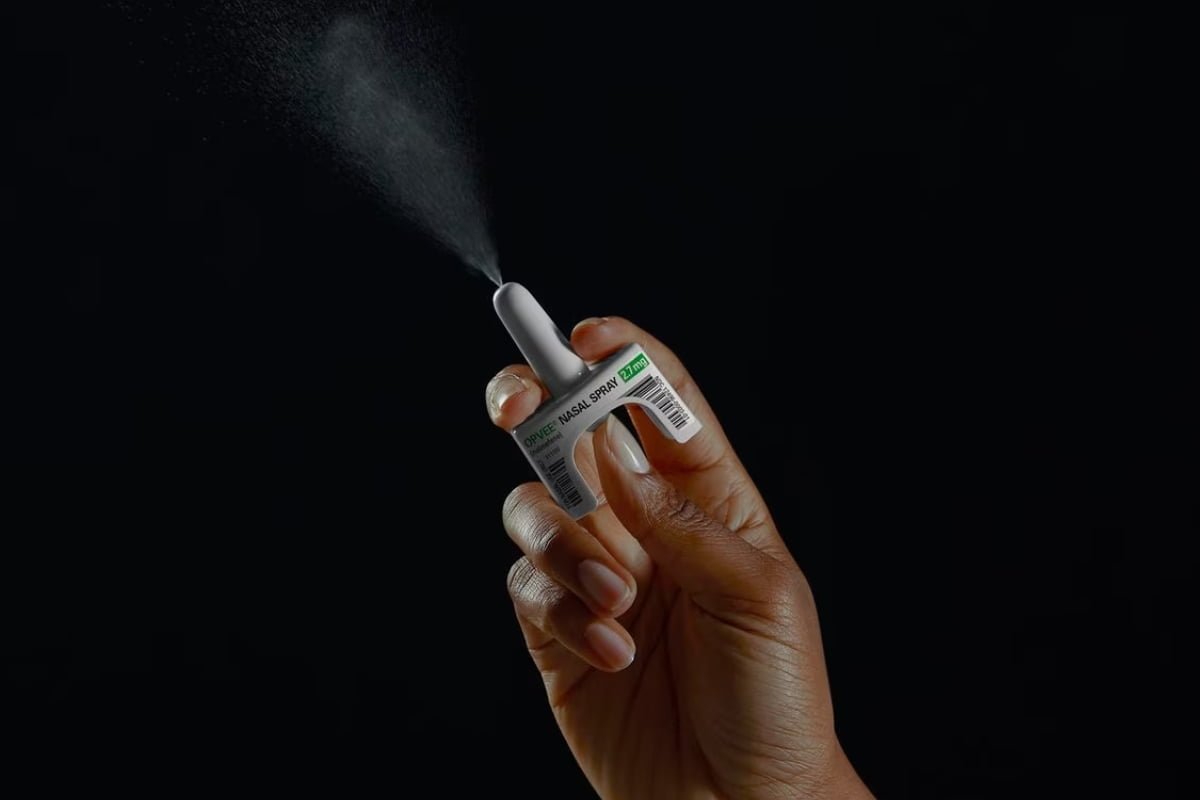
Indivior Receives FDA Approval for OPVEE (nalmefene)
Indivior’s OPVEE® (nalmefene) nasal spray has received FDA approval for the emergency treatment of opioid overdose in adults and pediatric ...
Epkinly: FDA-Approved Bispecific Antibody for DLBCL
On May 19, 2023, AbbVie announced that the U.S. Food and Drug Administration (FDA) granted approval to EPKINLYTM (epcoritamab-bysp) as ...

LEXICON ANNOUNCES FDA APPROVAL OF INPEFA (SOTAGLIFLOZIN) FOR TREATMENT OF HEART FAILURE
Lexicon Pharmaceuticals on May 26, 2023 announced that the US Food and Drug Administration (FDA) has approved INPEFA™ (sotagliflozin), a once-daily ...

ALLERGAN’S LATEST SKIN-SMOOTHING PRODUCT HAS RECEIVED FDA APPROVAL, FEATURING A UNIQUE DELIVERY MECHANISM
Allergan has recently obtained FDA approval for its groundbreaking skincare product, Skinvive, which is part of the Juvederm dermal filler ...
EPCORITAMAB RECEIVES FDA APPROVAL FOR RELAPSED/REFRACTORY DLBCL
The FDA has granted approval to epcoritamab-bysp (Epkinly), the first T-cell–engaging bispecific antibody, for the treatment of relapsed/refractory diffuse large ...
![THE U.S. FOOD AND DRUG ADMINISTRATION (FDA) HAS ISSUED A COMPLETE RESPONSE LETTER CONCERNING [VIC-]TRASTUZUMAB DUOCARMAZINE, A PRODUCT DEVELOPED BY BYONDIS.](https://pharmtales.com/wp-content/uploads/2023/06/FDA-Issues-Response-Letter-on-Byondis-Trastuzumab-Duocarmazine.jpg)
FDA Issues Response Letter on Byondis’ Trastuzumab Duocarmazine
Byondis B.V., a Dutch clinical-stage biopharmaceutical company specializing in precision medicines, has announced today that the U.S. Food and Drug ...







