FDA
PTC faces FDA hurdles for two drug candidates, delays filing
PTC Therapeutics finds itself in a regulatory whirlwind, as the latest third-quarter results reveal hurdles and setbacks impacting the biotech’s ...

UCB’s Swiss plant gets Form 483 from FDA over quality and records
UCB Farchim, a subsidiary of Belgian pharmaceutical company UCB, is facing regulatory scrutiny from the FDA. The FDA recently issued ...
AstraZeneca’s nasal flu vaccine nears FDA approval
AstraZeneca’s FluMist, a nasal spray flu vaccine with a two-decade history, is poised to potentially become available for self-administration as ...

Nkarta’s breakthrough cell therapy for lupus gets FDA approval after showing promise in cancer treatment
In a groundbreaking development, Nkarta is pushing the boundaries of cell therapy beyond the realm of cancer. The FDA has ...
Lawmakers demand answers from FDA and DEA on Adderall shortage crisis
As the nationwide shortage of medications like Adderall for attention-deficit/hyperactivity disorder (ADHD) continues to persist, a group of Congressional members ...

FDA Demands Further Safety Testing from Philips Amid Ongoing Concerns over CPAP Recall Handling
Over two years into Philips’ ongoing recall of millions of respiratory devices, the FDA has expressed dissatisfaction with the progress ...

Innate Pharma halts lymphomas trials after patient death, but key study continues
Innate Pharma has encountered a safety issue in its lymphoma program, resulting in the FDA imposing a partial clinical hold ...

FDA Approves 5-Minute Tests for Monitoring Humira and Remicade Doses in Inflammatory Bowel Disease Patients
The FDA has recently granted two de novo clearances to ProciseDx, marking a significant advancement in personalized medicine for patients ...

Pfizer’s Hospira Initiates Recall of 3 Drug Lots Due to Particulate Concerns
Hospira, a subsidiary of Pfizer, has taken the proactive step of issuing a voluntary recall for specific injectable medications, namely ...

Invitae gets FDA approval for blood test that detects hereditary cancer genes
Invitae has achieved a significant milestone with the FDA granting de novo clearance for its groundbreaking blood test. This test ...

Eli Lilly settles Whistleblower lawsuit over alleged quality issues
Settlement talks have been underway between pharmaceutical giant Eli Lilly and a former human resources officer, Amrit Mula, who blew ...

ImmunityBio cuts 50 jobs in California and Florida after FDA snubs its bladder cancer drug
Following an FDA rejection earlier this year, ImmunityBio has initiated its second round of layoffs in less than a year, ...

Mesoblast hopes to win FDA approval for remestemcel-L after meeting with regulators
Mesoblast, a biotech company based in Melbourne, Australia, is making determined strides toward securing FDA approval for its off-the-shelf therapy, ...

Novo Nordisk’s semaglutide facility in North Carolina faces FDA scrutiny over quality issues
Novo Nordisk’s shares faced a decline following reports that the FDA had raised concerns about manufacturing issues at the company’s ...

FDA issues guidance on single-trial evidence for drug approval
Biopharmaceutical companies seeking FDA approval based on a single clinical trial must provide comprehensive and transparent data, including both positive ...
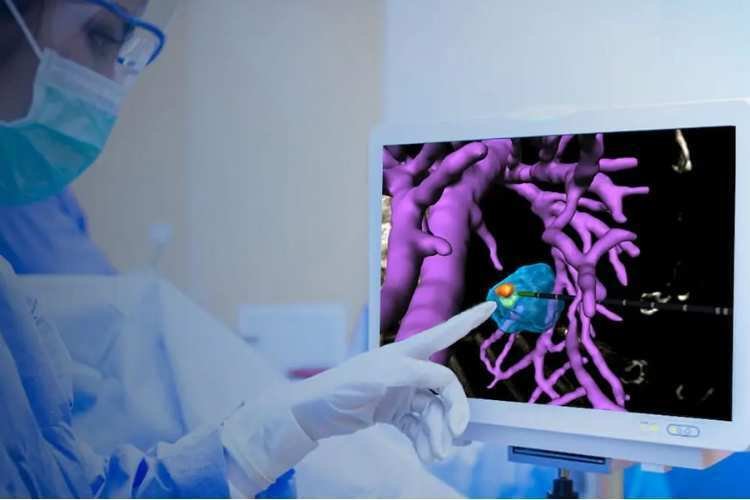
FDA approves world’s first MRI-compatible breast biopsy robot
Insight Medbotics has achieved FDA clearance for what it claims to be the world’s first surgical robot designed to operate ...

Rocket Pharma gets FDA green light for gene therapy trial, stock surges
Rocket Pharmaceuticals has achieved alignment with the FDA regarding the design of a pivotal phase 2 clinical trial for a ...

Madrigal’s NASH drug gets fast-track review from FDA, no panel needed
In the wake of a recent CEO transition, Madrigal Pharmaceuticals has disclosed that its nonalcoholic steatohepatitis (NASH) drug has been ...
FDA cracks down on 8 firms selling illegal eye drugs online
In a recent development, eight companies have come under scrutiny for allegedly producing unauthorized eye medications, according to the FDA’s ...

Ferring launches bladder cancer gene therapy Adstiladrin in US through early experience program
Ferring Pharmaceuticals achieved a significant milestone by administering its groundbreaking gene therapy, Adstiladrin, to its first commercial patient. This innovative ...

FDA panel votes against phenylephrine, a common decongestant drug in many cold medicines
For decades, the shelves of American pharmacies have been stocked with hundreds of oral decongestant products containing phenylephrine, promising relief ...

Neurocrine advances potential blockbuster drug for endometrial hyperplasia after phase 3 success
Neurocrine Biosciences is banking on its hyperplasia drug, crinecerfont, which analysts predict could be a blockbuster. The company has been ...
Merck showcases new data on sotatercept for PAH, awaits FDA decision
Merck & Co. has unveiled new clinical data on sotatercept, reinforcing the drug candidate’s safety and efficacy in the treatment ...

Astrazeneca Suffers Setback As FDA Rejects Ultomiris For Rare Autoimmune Disease
AstraZeneca’s plans to expand the use of Ultomiris in the United States have encountered a setback, as the FDA issued ...

Dräger Faces FDA Class I Recall on Carina Ventilators Due to Contaminant Concerns
Dräger, a German medical device manufacturer, is grappling with its fourth Class I recall by the FDA this year, this ...

FDA Advisors Favor Otsuka’s ReCor and Express Reservations on Medtronic’s Renal Denervation Therapies
During a two-day-long FDA meeting, a panel of advisors deliberated over competing proposals for novel renal denervation therapies, ultimately granting ...

FDA Grants Approval to Ipsen’s Sohonos Capsules, the First-Ever Treatment for Individuals with Fibrodysplasia Ossificans Progressiva
In 2019, Ipsen made a significant investment of $1 billion to acquire Clementia Pharmaceuticals and its rare disease drug, palovarotene. ...

Abortion Pill Restrictions Upheld by Federal Appeals Court
US Federal Appeals Court Partially Supports Limits on Access to Medical Abortion Pill, Setting Stage for Possible Supreme Court Decision. ...

GSK’s Arexvy Takes Lead in RSV Vaccine Race, Now Accessible at Leading US Pharmacies
In the competitive landscape of the respiratory syncytial virus (RSV) vaccine market, GlaxoSmithKline (GSK) stands out with its well-timed strides. ...








