Elevidys

Catalent Braces for Potential Revenue Setback Following Sarepta’s Gene Therapy Trial Disappointment
Catalent, having overcome the challenges posed by the pandemic, now faces another potential setback in the form of what analysts ...

Sarepta Therapeutics Sells Rare Pediatric Disease Priority Review Voucher for $102 Million to Boost Research and Development
Source – Sarepta Therapeutics On July 5, 2023, Sarepta Therapeutics, a leading company in precision genetic medicine for rare diseases, ...
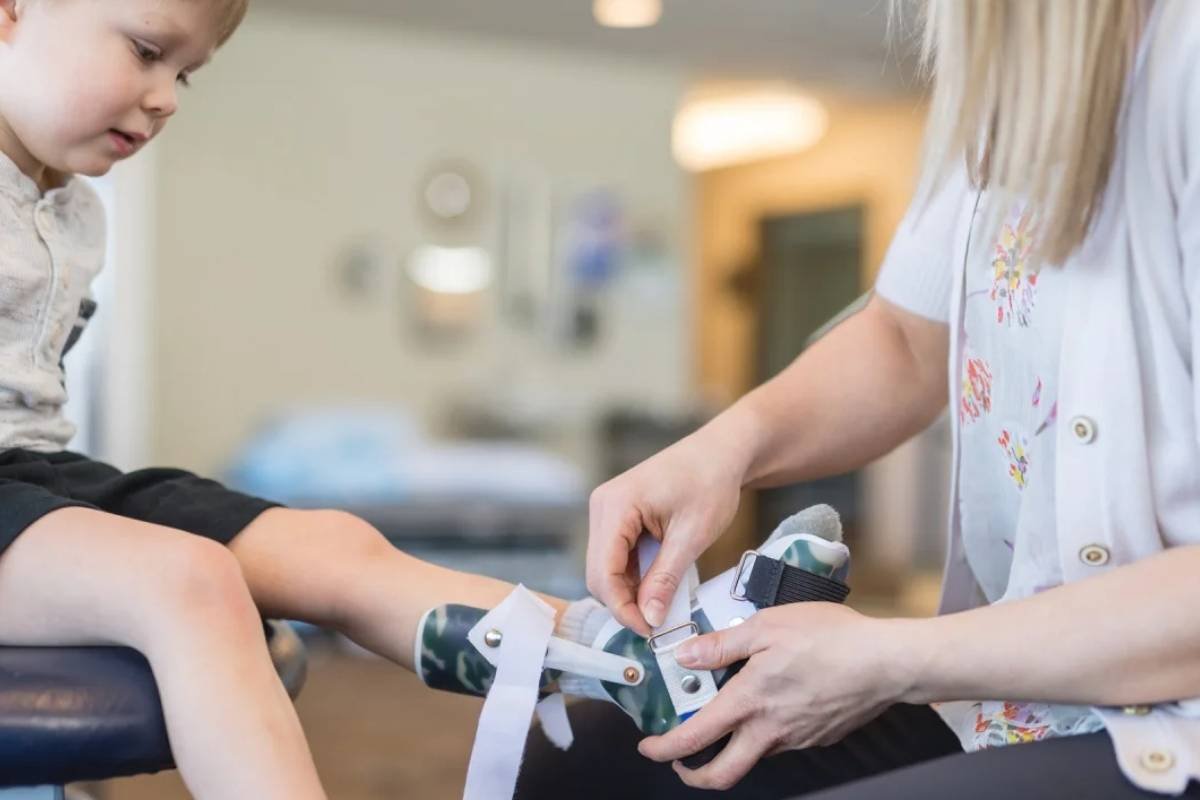
FDA’s Peter Marks Overrides Reviewers’ Rejection to Approve Sarepta’s Gene Therapy for Duchenne Muscular Dystrophy
Despite the FDA review teams initially leaning against approving Sarepta’s Duchenne muscular dystrophy (DMD) gene therapy, a memo reveals that ...

Sarepta Therapeutics Makes History with FDA Approval of Elevidys, the First Gene Therapy for Duchenne Muscular Dystrophy
Source – Sarepta Therapeutics On June 22, 2023, Sarepta Therapeutics made an announcement regarding the accelerated approval granted by the ...

Sarepta’s Breakthrough DMD Gene Treatment, Elevidys, Triumphs FDA Hurdles at $3.2M
The speedy approval of Sarepta’s Duchenne muscular dystrophy (DMD) gene therapy has been achieved after several delays and a close ...







