Roche’s Columvi (glofitamab) receives FDA accelerated approval for treatment of Large b Cell Lymphoma, sparking a rivalry with AbbVie and Genmab’s Epkinly
dIffuse Large b Cell Lymphoma Treatment
In the dynamic landscape of LBCL, Roche has emerged as a formidable contender with the FDA’s accelerated approval of Columvi. This bispecific T-cell engager, targeting CD20xCD3, is set to challenge Epkinly, recently approved by AbbVie and Genmab. While both drugs aim to combat the same disease, Columvi’s label focuses primarily on (DLBCL), leaving out high-grade LBCL despite promising results from its Phase II trial.
The rivalry between Columvi and Epkinly goes beyond their mechanisms of action. Epkinly gains an edge in convenience with its under-the-skin injection administration, while Columvi currently requires intravenous infusion. Nevertheless, Roche is actively exploring a subcutaneous formulation for Columvi, aiming to match Epkinly’s user-friendly approach.
What sets Columvi apart, however, is its distinctive fixed duration of treatment. Unlike the traditional approach of treatment until progression, Columvi offers patients a treatment-free interval, reducing the likelihood of long-term side effects. This novel strategy has drawn attention, with Ginna Laport, Genentech’s global head of lymphoma and chronic lymphocytic leukemia development, highlighting its potential benefits.
Comparing the efficacy of Columvi and Epkinly, both drugs demonstrate promising response rates. Columvi’s FDA label reveals a 56% overall response rate in DLBCL patients, with 43% achieving complete remission. Epkinly, on the other hand, boasts a slightly higher response rate of 61%, with 38% of patients achieving complete remission. While Columvi appears to be catching up to Epkinly in terms of efficacy, longer follow-up data is required to assess the durability of response and the potential for sustained benefits.
It’s important to note that both drugs are associated with cytokine release syndrome (CRS), an immune-related side effect. Columvi reports a CRS rate of 70%, with 4.1% experiencing grade 3 or 4 CRS. In comparison, Epkinly’s trial results indicate a lower CRS rate of 51%, with 2.5% experiencing grade 3 CRS.
While the FDA has granted accelerated approval for Columvi, further confirmatory data is necessary. Roche is currently conducting the phase 3 STARGLO trial, which compares Columvi plus chemotherapy to a Rituxan-chemotherapy combination in second-line (DLBCL) patients ineligible for stem cell transplant. The results of this trial will provide additional insights into the efficacy and safety of Columvi.
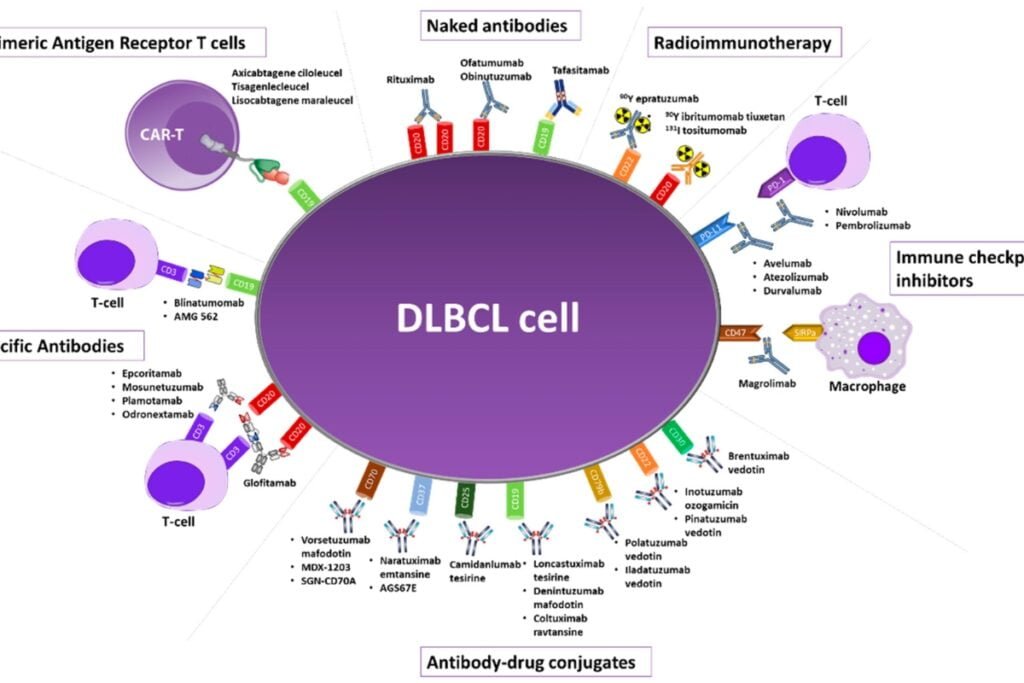
Roche’s foray into the LBCL arena doesn’t end with Columvi. The company has already secured FDA approvals for Lunsumio as a third-line therapy for follicular lymphoma and Polivy, an antibody-drug conjugate, as part of a first-line therapy for DLBCL. Roche’s ongoing SUNMO trial explores the potential of combining Lunsumio with Polivy in second-line DLBCL.
As the rivalry between Columvi and Epkinly unfolds, the quest for more effective and safer treatments for LBCL continues. These innovative therapies offer hope to patients and drive progress in the field, ultimately bringing us closer to conquering this challenging disease.