Pharma
Stay updated with the latest happenings in the pharmaceutical industry. Get insights into drug development, innovations, regulatory updates, and emerging trends. Discover the advancements shaping the future of healthcare. Explore the world of pharma with our comprehensive coverage at Pharmtales.com.

How to Deal with Enlarged Prostate in Young Males: Symptoms, Causes and Treatment Options
If you are a young man with trouble peeing, you might think you have a urinary infection or a kidney ...

DATOPOTAMAB DERUXTECAN MEETS DUAL PRIMARY ENDPOINT IN TROPION-LUNG01 PHASE III TRIAL FOR ADVANCED NON-SMALL CELL LUNG CANCER
On July 3, 2023, AstraZeneca presented encouraging top-line results from the TROPION-Lung01 Phase III trial that demonstrated the positive efficacy ...
SELINEXOR AND RUXOLITINIB’S PHASE III TRIAL IN JAKI-NAIVE MYELOFIBROSIS BEGINS
Source – Karyopharm Therapeutics Karyopharm Therapeutics has initiated a Phase III clinical trial (NCT04562389) to evaluate the effectiveness and safety ...
Pembrolizumab demonstrate effectiveness beyond five years as a first-line treatment for advanced NSCLC with PD-L1 expression
First-line treatment with pembrolizumab (Keytruda) has demonstrated long-term survival benefits and durable responses in patients with PD-L1-positive, locally advanced or ...
CMS introduces adjustments to Medicare price negotiation while maintaining a strong stance
The Centers for Medicare and Medicaid Services (CMS) has made some minor adjustments to its plan allowing Medicare to negotiate ...
Gilead and Teva were acquitted in a $3.6 billion trial involving alleged ‘pay-for-delay’ schemes
Gilead Sciences and Teva have been exonerated by a California court jury in a lawsuit accusing them of colluding to ...

Novartis Divests Xiidra and Other Ophthalmology Assets to Bausch + Lomb for $2.5 Billion
Source – Novartis In a strategic move to concentrate on its core areas, Novartis has decided to transfer the dry ...

Fezolinetant’s Phase IIIb Trial Demonstrates Promising Topline Results in Treating Menopause-related Vasomotor Symptoms (VMS)
Source – Astellas Pharma Astellas Pharma recently announced positive preliminary findings on June 27, 2023, from the Phase IIIb DAYLIGHT ...
Sandoz Enters US Immunology Market with Launch of High-Concentration Formulation Hyrimoz
Source – Novartis On July 1, 2023, Sandoz made an announcement regarding the availability of their biosimilar Hyrimoz (adalimumab-adaz) injection ...
Novel BRG1/BRM Inhibitor in Metastatic Uveal Melanoma Shows Safety and Preliminary Efficacy
Source – Foghorn Therapeutics Foghorn Therapeutics has highlighted the safety and tolerability of FHD-286 in metastatic uveal melanoma (mUM) through ...
Quaratusugene Ozeplasmid Plasmid Plus Atezolizumab in ES-SCLC Receives FTD from FDA
Source – Genprex On June 28, 2023, Genprex has announced that the FDA has granted fast track designation (FTD) to ...
Briqulimab Phase I Study Doses First Patient With Lower-Risk MDS
Source – Jasper Therapeutics Jasper Therapeutics has initiated a Phase I trial (NCT05903274) to evaluate the use of briquilimab as ...
Clinical Activity Is Provoked by Teclistamab Triplet in R/R Multiple Myeloma
The Phase Ib (NCT04722146) MajesTEC-2 trial demonstrated promising clinical activity in patients with relapsed/refractory multiple myeloma (RRMM) when combining teclistamab ...
First cell treatment for type I diabetes is approved by the FDA
Source – FDA On June 29, 2023, CellTrans achieved a significant milestone by securing the first-ever FDA approval for a ...
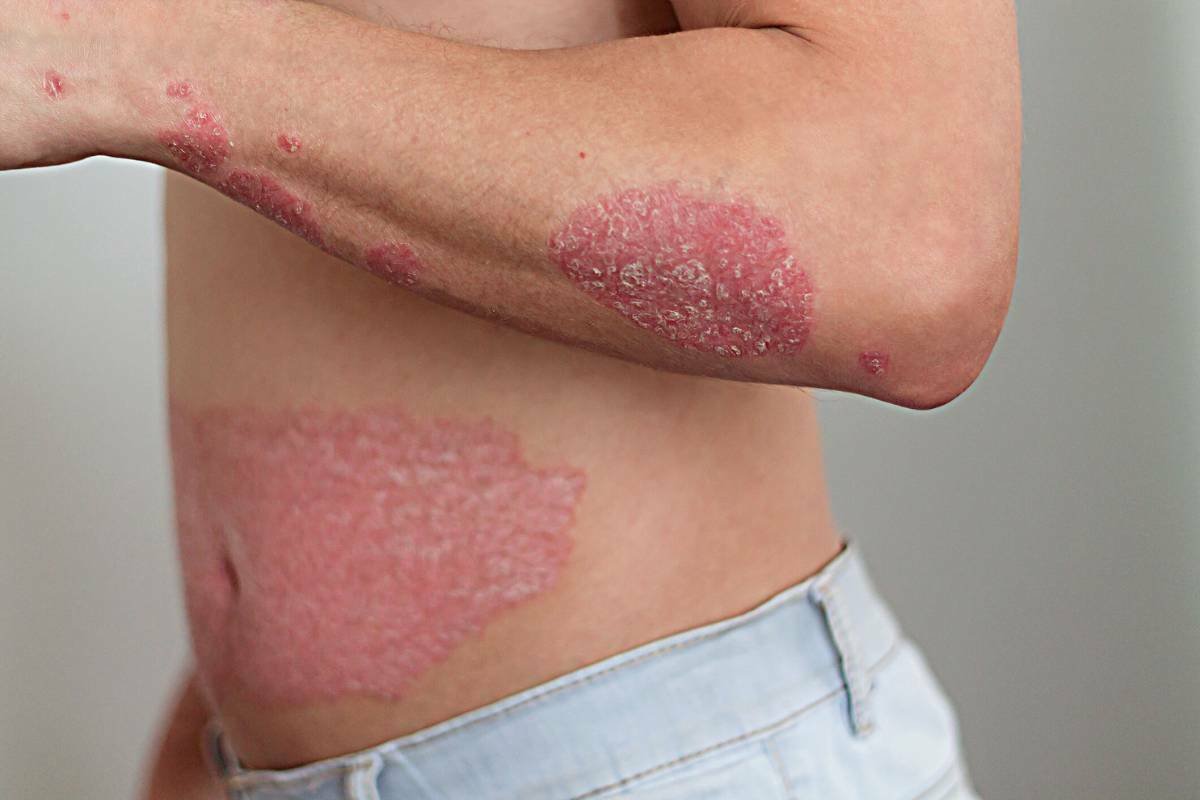
BMS’ Sotyktu is approved for psoriasis usage by the NHS
Source: Bristol-Myers Squibb Just weeks after receiving approval in the UK, Bristol-Myers Squibb’s novel oral therapy for psoriasis, SotyKtu, has ...
Missed trial procedures, made-up emails, and an unsuccessful endpoint make a mess Alzheimer’s agitation readout from BioXCel
Source – BioXcel Therapeutics On June 29, 2023, a potential breakthrough in the treatment of agitation episodes in Alzheimer’s disease ...
BioMarin’s Roctavian: FDA Approves Hemophilia A Gene Therapy with Positive Reception from Physician
Source – BioMarin BioMarin’s Roctavian, a gene therapy for severe hemophilia A, has received FDA approval after initial rejection in ...

Sigilon Therapeutics will be acquired by Lilly
Source – Eli Lilly On June 29, 2023 Eli Lilly and Company and Sigilon Therapeutics entered into a definitive agreement, ...
Imfinzi + Imjudo: Unprecedented 4-Year Survival in Advanced Liver Cancer
Source – AstraZeneca On 29 June 2023, new findings from the HIMALAYA Phase III trial revealed that AstraZeneca’s Imfinzi (durvalumab) ...
Influenza Variant Virus in Brazil: What You Need to Know
What Is the Influenza Variant Virus in Brazil and How to Protect Yourself Influenza is a viral infection that affects ...
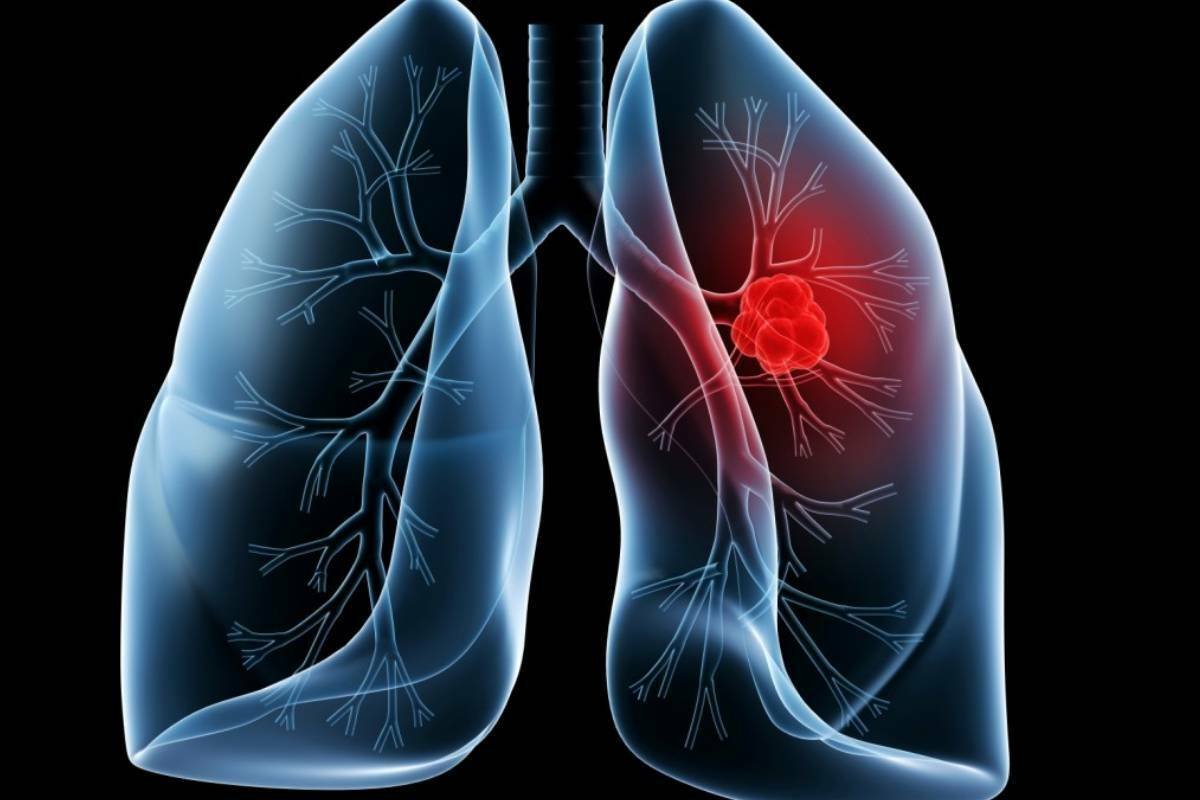
Lung Cancer Stage 3 Survival Rate: Everything You Need to Know
A Comprehensive Guide to Lung Cancer Stage 3 Survival Rate and Treatment Options This blog will reveal all about lung ...

Prostate Cancer Treatment: What Every Man Should Know About and How to Treat It
Everything You Need to Know About Prostate Cancer Treatment and Prevention A lot of men are affected by prostate cancer, ...
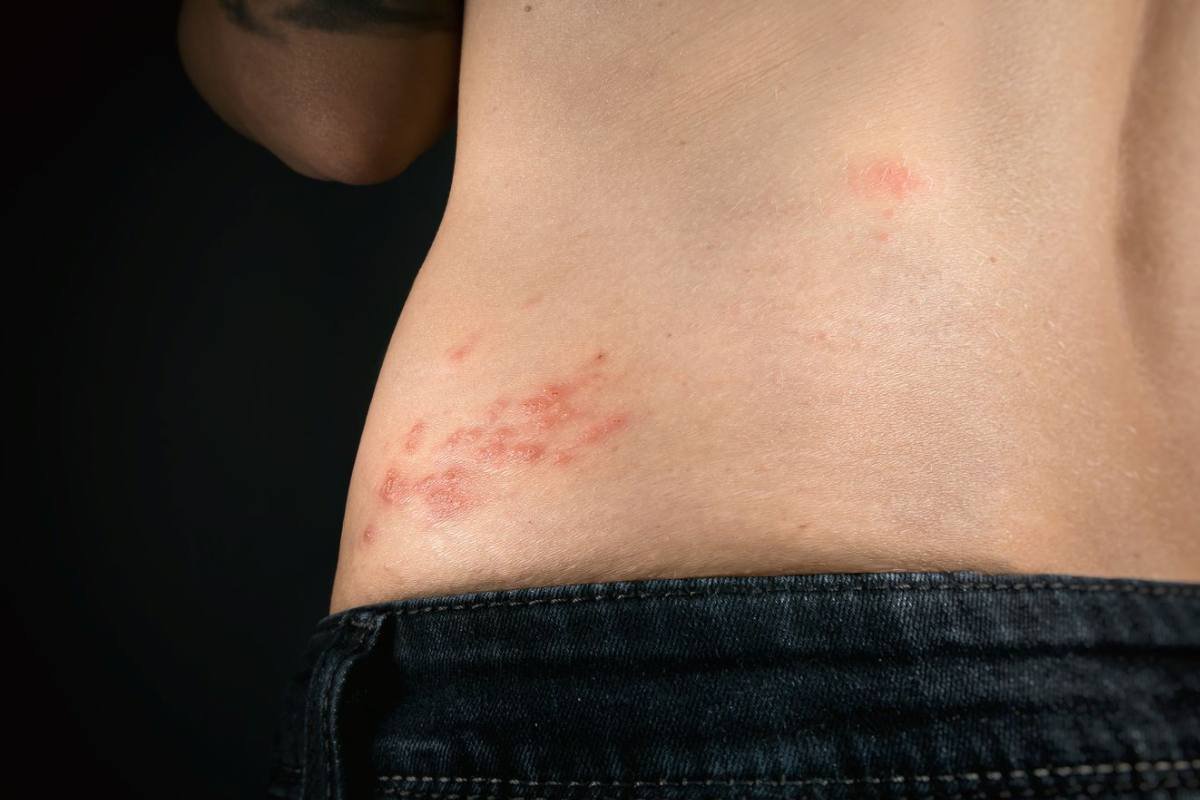
Shingrix Granted Approval by Japan’s Ministry of Health Labour and Welfare for Shingles Prevention in At-Risk Individuals 18 Years and Older
Source – GSK On 26 June 2023, GSK announced that the Japanese Ministry of Health, Labour and Welfare (MHLW) has ...
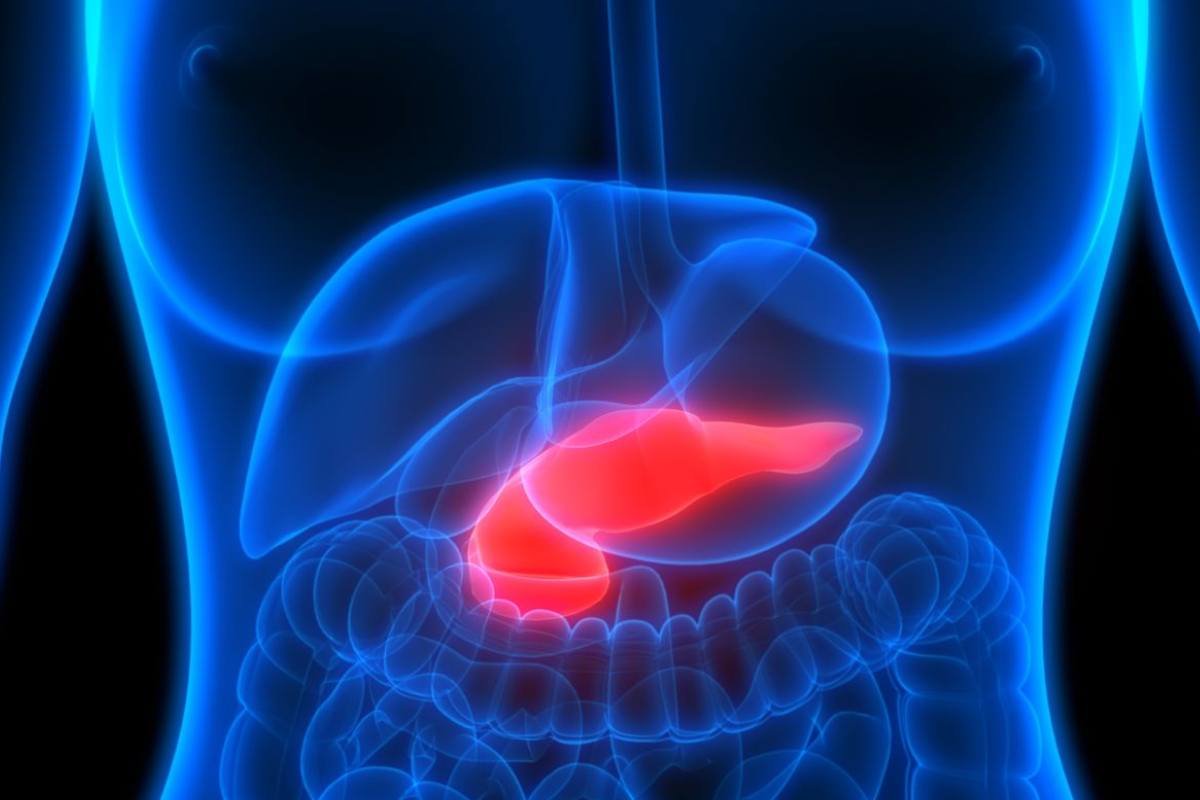
VCN-01 Plus Chemotherapy Receives FDA Orphan Drug Designation for Early PDAC Treatment
Source – Theriva Biologics On 28June 2023, the FDA has granted Orphan Drug Designation (ODD) to VCN-01 in combination with ...

Bellus Health Acquired by GSK is complete
Source – GSK On 28 June 2023, GSK and BELLUS Health announced the completion of GSK’s acquisition of BELLUS through ...

As a result of production issues, the FDA rejected Eton Pharma’s proposed therapy for methanol poisoning
Source – Eton Pharmaceuticals Eton Pharmaceuticals’ dehydrated alcohol injection did not receive approval from the FDA and instead received a ...
Reata Pharmaceuticals Begins Launching Its First Commercial Product Skyclarys with Approved Supplement
Source – Reata Pharmaceuticals After receiving approval in the spring, Reata Pharmaceuticals has been eagerly awaiting the launch of its ...

Investor NexPoint has threatened to prevent the $462 million sale of Paratek
Source – Paratek Pharmaceuticals On 29 June, Paratek Pharmaceuticals is scheduled to hold its annual meeting, but a contentious situation ...

Dementia Solutions: Eisai, Gates, UK Health & Univ. Edinburgh
Source – Eisai On June 29, 2023, Eisai, Gates Ventures, Health Data Research UK, LifeArc, and The University of Edinburgh ...