Latest News
Get real-time updates on the latest news and developments in the pharmaceutical world. Stay informed about new drug launches, research findings, regulatory changes, and industry updates. Explore a wide range of topics and stay connected with the rapidly evolving world of pharma.
FDA rejects Amneal’s Parkinson’s drug, $500M peak sales goal unattainable
Source – Amneal Pharmaceuticals On July 3, 2023, Amneal Pharmaceuticals’ plans to launch its extended-release Parkinson’s disease treatment, IPX203, by ...
Roche withdraws Gavreto approval, citing study impracticality
Roche’s cancer drug Gavreto was withdrawn for the treatment of advanced RET-mutant medullary thyroid cancer, following discussions with the FDA. ...

Sanofi teams up with local pharma firms for vaccine production in Saudi Arabia
Following recent developments by AstraZeneca and National Resilience in the United Arab Emirates, Sanofi has emerged as the next major ...
FDA Approves Cyclophosphamide Injection NDA for Multiple Cancers
Source – Nevakar Injectables On July 3, 2023 the FDA has approved a new drug application (NDA) for 200-mg/mL vials ...
Zenocutuzumab receives Breakthrough Designation from the FDA for NRG1+ Pancreatic Cancer
Source – Merus On July 5, The FDA has granted breakthrough therapy designation to zenocutuzumab (MCLA-128) as a treatment for ...
Janssen rejoices at JNJ-2113A’s successful psoriasis study findings
Source – Johnson & Johnson On July 4, 2023, Janssen, a subsidiary of Johnson & Johnson announced positive initial results ...
For its updated Covid-19 vaccine, Moderna submits a regulatory application to the European Medicines Agency
Source – Moderna On July 3, 2023 Moderna announced the submission of a regulatory application to the European Medicines Agency ...

Healthcare bucks the trend with a 2-year M&A high
On July 4, Data from LSEG Deals Intelligence revealed that the healthcare sector experienced a notable 35% increase in deals, ...

MDMA and psilocybin were originally prescribed for depression and PTSD in Australia
Australia made history on July 3rd by becoming the first country to allow psychiatrists to prescribe MDMA, commonly known as ...

DATOPOTAMAB DERUXTECAN MEETS DUAL PRIMARY ENDPOINT IN TROPION-LUNG01 PHASE III TRIAL FOR ADVANCED NON-SMALL CELL LUNG CANCER
On July 3, 2023, AstraZeneca presented encouraging top-line results from the TROPION-Lung01 Phase III trial that demonstrated the positive efficacy ...
SELINEXOR AND RUXOLITINIB’S PHASE III TRIAL IN JAKI-NAIVE MYELOFIBROSIS BEGINS
Source – Karyopharm Therapeutics Karyopharm Therapeutics has initiated a Phase III clinical trial (NCT04562389) to evaluate the effectiveness and safety ...
CMS introduces adjustments to Medicare price negotiation while maintaining a strong stance
The Centers for Medicare and Medicaid Services (CMS) has made some minor adjustments to its plan allowing Medicare to negotiate ...
Gilead and Teva were acquitted in a $3.6 billion trial involving alleged ‘pay-for-delay’ schemes
Gilead Sciences and Teva have been exonerated by a California court jury in a lawsuit accusing them of colluding to ...

Novartis Divests Xiidra and Other Ophthalmology Assets to Bausch + Lomb for $2.5 Billion
Source – Novartis In a strategic move to concentrate on its core areas, Novartis has decided to transfer the dry ...

Fezolinetant’s Phase IIIb Trial Demonstrates Promising Topline Results in Treating Menopause-related Vasomotor Symptoms (VMS)
Source – Astellas Pharma Astellas Pharma recently announced positive preliminary findings on June 27, 2023, from the Phase IIIb DAYLIGHT ...
Sandoz Enters US Immunology Market with Launch of High-Concentration Formulation Hyrimoz
Source – Novartis On July 1, 2023, Sandoz made an announcement regarding the availability of their biosimilar Hyrimoz (adalimumab-adaz) injection ...
Novel BRG1/BRM Inhibitor in Metastatic Uveal Melanoma Shows Safety and Preliminary Efficacy
Source – Foghorn Therapeutics Foghorn Therapeutics has highlighted the safety and tolerability of FHD-286 in metastatic uveal melanoma (mUM) through ...
Quaratusugene Ozeplasmid Plasmid Plus Atezolizumab in ES-SCLC Receives FTD from FDA
Source – Genprex On June 28, 2023, Genprex has announced that the FDA has granted fast track designation (FTD) to ...
Briqulimab Phase I Study Doses First Patient With Lower-Risk MDS
Source – Jasper Therapeutics Jasper Therapeutics has initiated a Phase I trial (NCT05903274) to evaluate the use of briquilimab as ...
Clinical Activity Is Provoked by Teclistamab Triplet in R/R Multiple Myeloma
The Phase Ib (NCT04722146) MajesTEC-2 trial demonstrated promising clinical activity in patients with relapsed/refractory multiple myeloma (RRMM) when combining teclistamab ...
First cell treatment for type I diabetes is approved by the FDA
Source – FDA On June 29, 2023, CellTrans achieved a significant milestone by securing the first-ever FDA approval for a ...
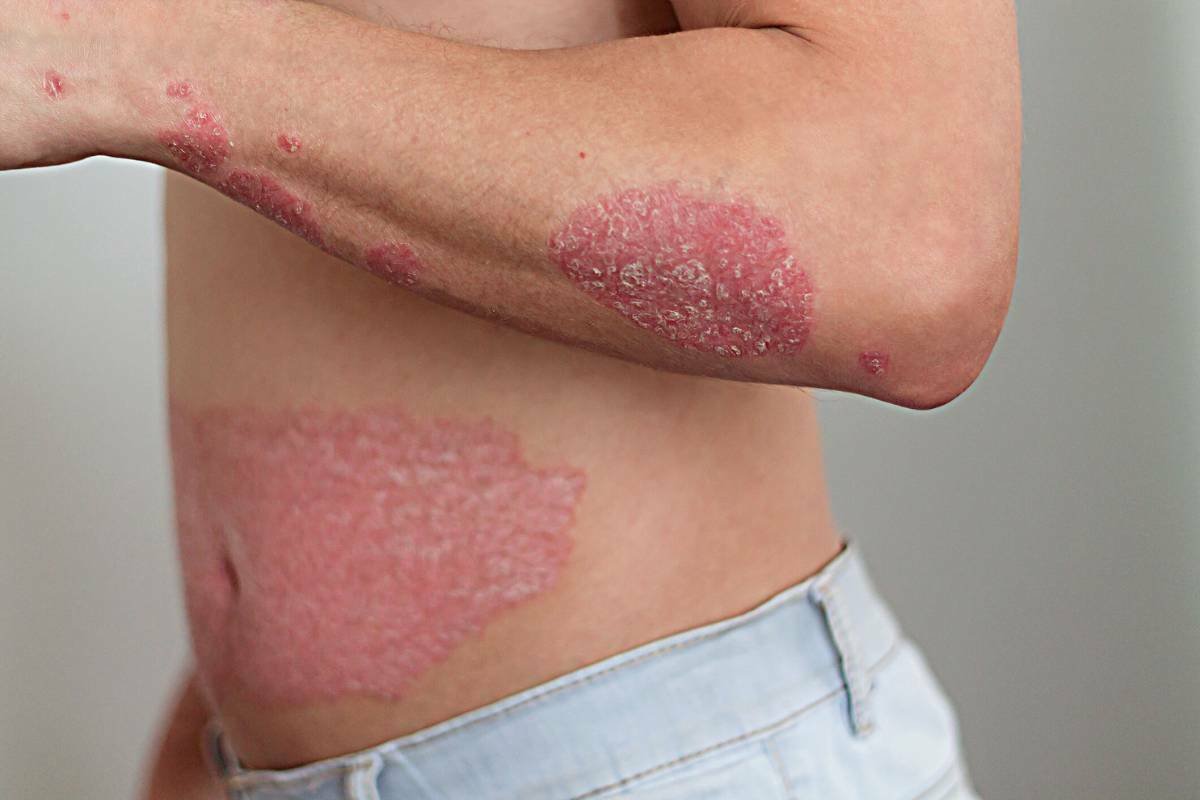
BMS’ Sotyktu is approved for psoriasis usage by the NHS
Source: Bristol-Myers Squibb Just weeks after receiving approval in the UK, Bristol-Myers Squibb’s novel oral therapy for psoriasis, SotyKtu, has ...
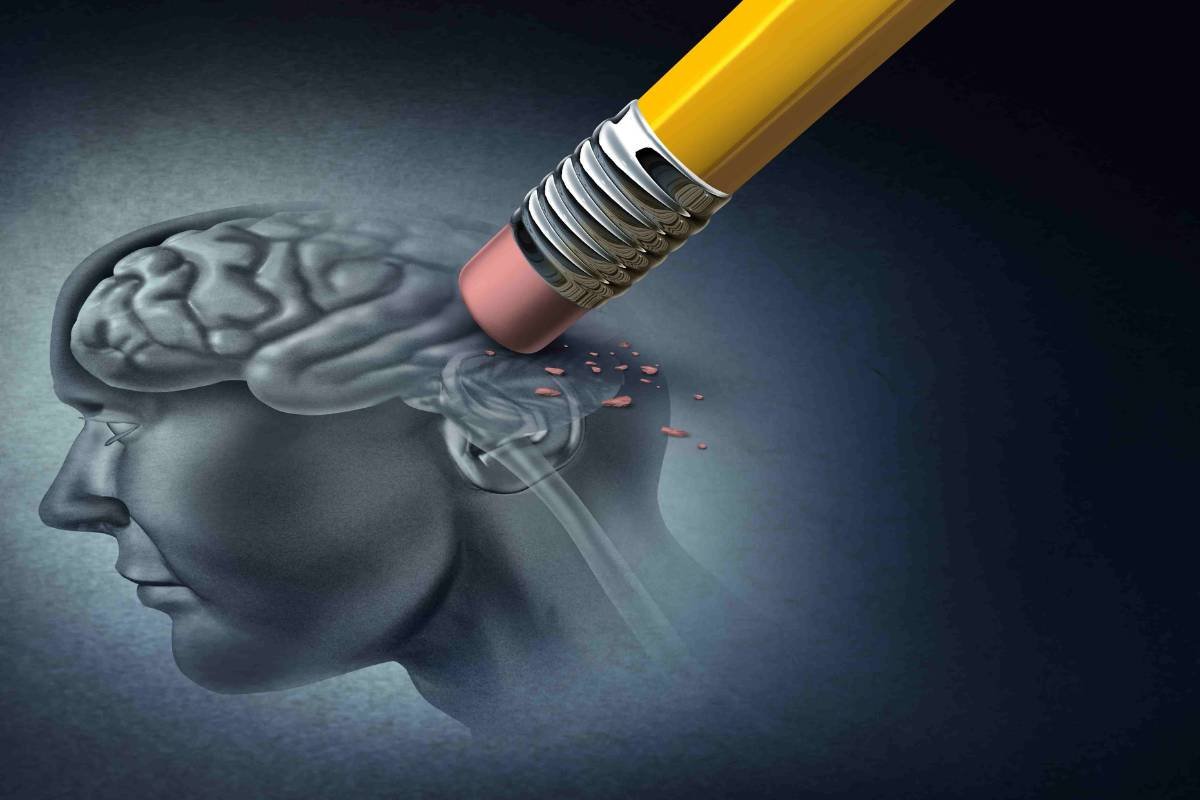
Missed trial procedures, made-up emails, and an unsuccessful endpoint make a mess Alzheimer’s agitation readout from BioXCel
Source – BioXcel Therapeutics On June 29, 2023, a potential breakthrough in the treatment of agitation episodes in Alzheimer’s disease ...
BioMarin’s Roctavian: FDA Approves Hemophilia A Gene Therapy with Positive Reception from Physician
Source – BioMarin BioMarin’s Roctavian, a gene therapy for severe hemophilia A, has received FDA approval after initial rejection in ...

Sigilon Therapeutics will be acquired by Lilly
Source – Eli Lilly On June 29, 2023 Eli Lilly and Company and Sigilon Therapeutics entered into a definitive agreement, ...
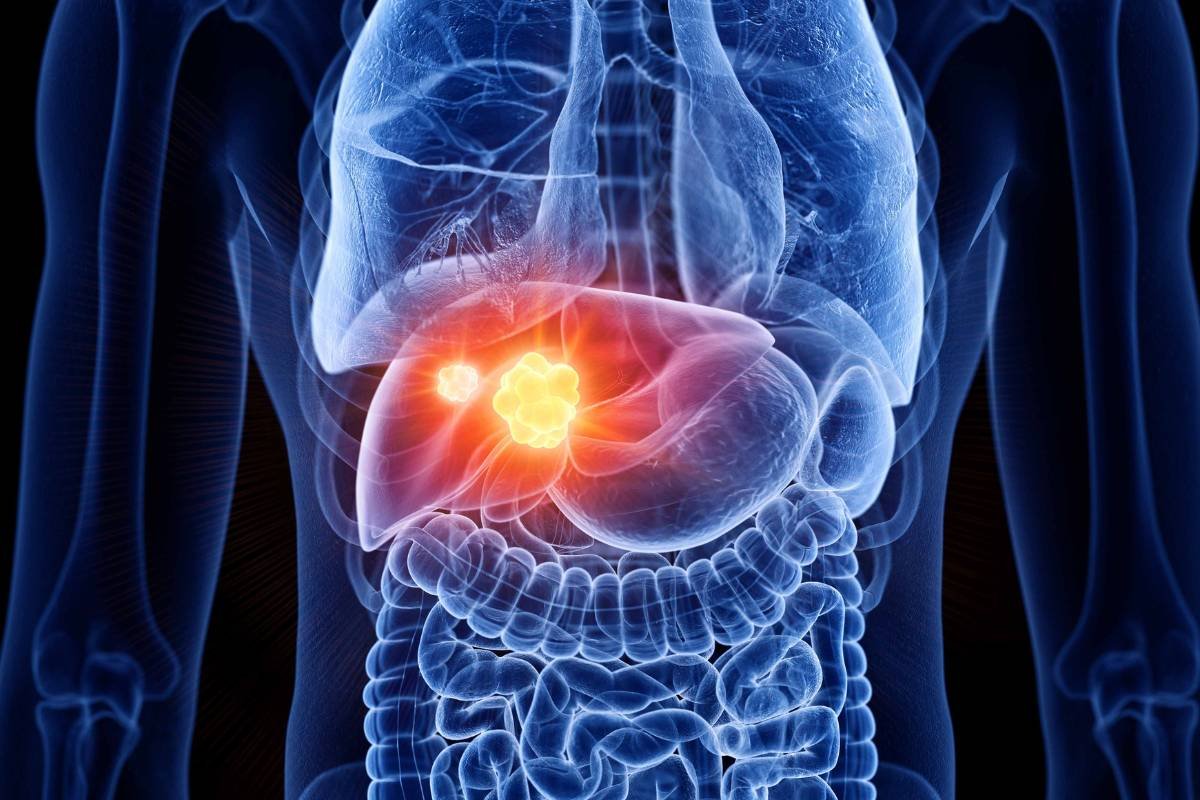
Imfinzi + Imjudo: Unprecedented 4-Year Survival in Advanced Liver Cancer
Source – AstraZeneca On 29 June 2023, new findings from the HIMALAYA Phase III trial revealed that AstraZeneca’s Imfinzi (durvalumab) ...
Shingrix Granted Approval by Japan’s Ministry of Health Labour and Welfare for Shingles Prevention in At-Risk Individuals 18 Years and Older
Source – GSK On 26 June 2023, GSK announced that the Japanese Ministry of Health, Labour and Welfare (MHLW) has ...
VCN-01 Plus Chemotherapy Receives FDA Orphan Drug Designation for Early PDAC Treatment
Source – Theriva Biologics On 28June 2023, the FDA has granted Orphan Drug Designation (ODD) to VCN-01 in combination with ...

Bellus Health Acquired by GSK is complete
Source – GSK On 28 June 2023, GSK and BELLUS Health announced the completion of GSK’s acquisition of BELLUS through ...