Blog

Huma Received FDA Class II clearance for the SaMD platform
Source – HUMA With the FDA’s Class II clearance of its Software as a Medical Device (SaMD) disease management platform, ...

Almirall plans to expand its pipeline with €200 million in capital
Source – Almirall Almirall has completed a €200 million share capital issue, some of which will be used to finalize ...

Valneva Preparing for the FDA’s decision on the chikungunya vaccine
Just a few months away from the FDA making a judgment on Valneva’s marketing application for the chikungunya vaccine VLA1553, ...

Merck wins patent case for BRIDION in US District Court
Source- Merck On June 13, 2023 the US District Court for the District of New Jersey found in favor of ...

FDA authorizes the first medication for functional constipation in children
Source- FDA Pediatric patients aged 6 to 17 may use Linzess (linaclotide) pills to treat functional constipation. The first therapy ...

At EASL 2023, Merck will present data on the investigational GLP-1/glucagon receptor co-agonist Efinopegdutide (MK-6024) in patients with non-alcoholic fatty liver disease (NAFLD)
Source- Merck NJ-RAHWAY Efinopegdutide (MK-6024), an investigational GLP-1/glucagon receptor co-agonist, has new findings that have been accepted for oral presentation ...
In order to provide access to cervical cancer screening tools in low- and lower-middle income countries, Roche received WHO prequalification for the Cobas HPV test
Source – Roche On June 13, 2023, the World Health Organisation (WHO) prequalified the Cobas Human Papillomavirus (HPV) test for ...

Futura receives FDA approval for OTC treatment for erectile dysfunction
Source- Futura Medical The first business in the US to provide a topical erectile dysfunction medication to the market without ...

In the US, patients with advanced HR-positive breast cancer are given priority review for the combination of capivasertib and Faslodex
Source- Astrazeneca The US Food and Drug Administration (FDA) has accepted and granted Priority Review to AstraZeneca’s New Drug Application ...

For the treatment of people with refractory generalized myasthenia gravis, Soliris has received approval in China
Source- Astrazeneca For the treatment of adult patients with refractory generalized myasthenia gravis (gMG) and anti-acetylcholine receptor (AChR) antibody positivity, ...
Novartis has agreed to purchase Chinook Therapeutics for USD 3.2 billion upfront (USD 40 per share), strengthening its new medicines strategy and renal portfolio in the process
On 12 June 2023 Novartis revealed that it has reached a deal to purchase Chinook Therapeutics, a clinical-stage biopharmaceutical business ...
SAR’579/IPH6101 Granted FDA Fast Track Designation for Hematological Malignancies
Source: Innate Pharma On June 6, 2023, the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation to ...

Innate Pharma Showcases Breakthrough Tetra-Specific ANKET NK Cell Engager IPH6501 at the EHA 2023 Congress
Source: Innate Pharma On June 10, 2023, Innate Pharma, a biopharmaceutical company, highlights the latest preclinical findings on IPH6501, a ...

Kura Oncology Unveils Promising Clinical Results of Ziftomenib, at the 2023 European Hematology Association (EHA) Congress
Source – Kura Oncology Kura Oncology presented updated clinical data for its menin inhibitor, ziftomenib, at the 2023 European Hematology ...
FDA Advisory Committee Unanimously Recommends Nirsevimab for Preventing RSV Lower Respiratory Tract Disease in Infants
Source: AstraZeneca The FDA’s Antimicrobial Drugs Advisory Committee (AMDAC) has voted unanimously, with a 21 to 0 decision, affirming the ...
Danicopan Shows Promise as an Add-On to Ultomiris or Soliris, Enhancing Hemoglobin Levels and Disease Control in PNH Patients
Source – AstraZeneca Promising outcomes emerged from the pivotal Phase III ALPHA trial, revealing that danicopan, an investigational oral Factor ...

Novartis Survey Challenges Perception of CML as a Resolved Disease, Revealing Ongoing Impact and Need for Attention
Source: Novartis On June 9, 2023, Novartis reveals CML (chronic myeloid leukemia) SUN Results at 2023 European Hematology Association (EHA) ...

European Commission Approves COSENTYX as First and Only IL-17A Inhibitor for Hidradenitis Suppurativa
Source: Novartis On June 1, 2023, Novartis announced that the European Commission (EC) has granted approval for the use of ...

FDA Advisory Committee Vote Validates LEQEMBI Alzheimer’s Treatment
Source: Eisai On June 9, 2023, the Peripheral and Central Nervous System Drugs Advisory Committee (PCNS) of the US Food ...

AstraZeneca Enters $2 Billion Deal with Quell for Treg Cell Therapy
British start-up Quell Therapeutics has secured a collaboration with AstraZeneca focused on cell therapies for autoimmune diseases, with an upfront ...
FDA Sets December Deadline for Vertex and CRISPR’s Sickle Cell Therapy
The US Food and Drug Administration (FDA) has initiated a priority review of exagamglogene autotemcel (exa-cel), a therapy developed by ...

Samsung Biologics Secures $411 Million Long-Term Deal with Pfizer for Biosimilar Production
South Korea’s Samsung Biologics has recently entered into another significant manufacturing agreement, this time with Pfizer, worth $411 million. The ...

Sandoz Aims for Success in Biosimilars, Forecasts $3 Billion in Sales from Pipeline Products in Next 5 Years
Sandoz, the generics and biosimilars division of Novartis, is set to become an independent company and is determined to reverse ...
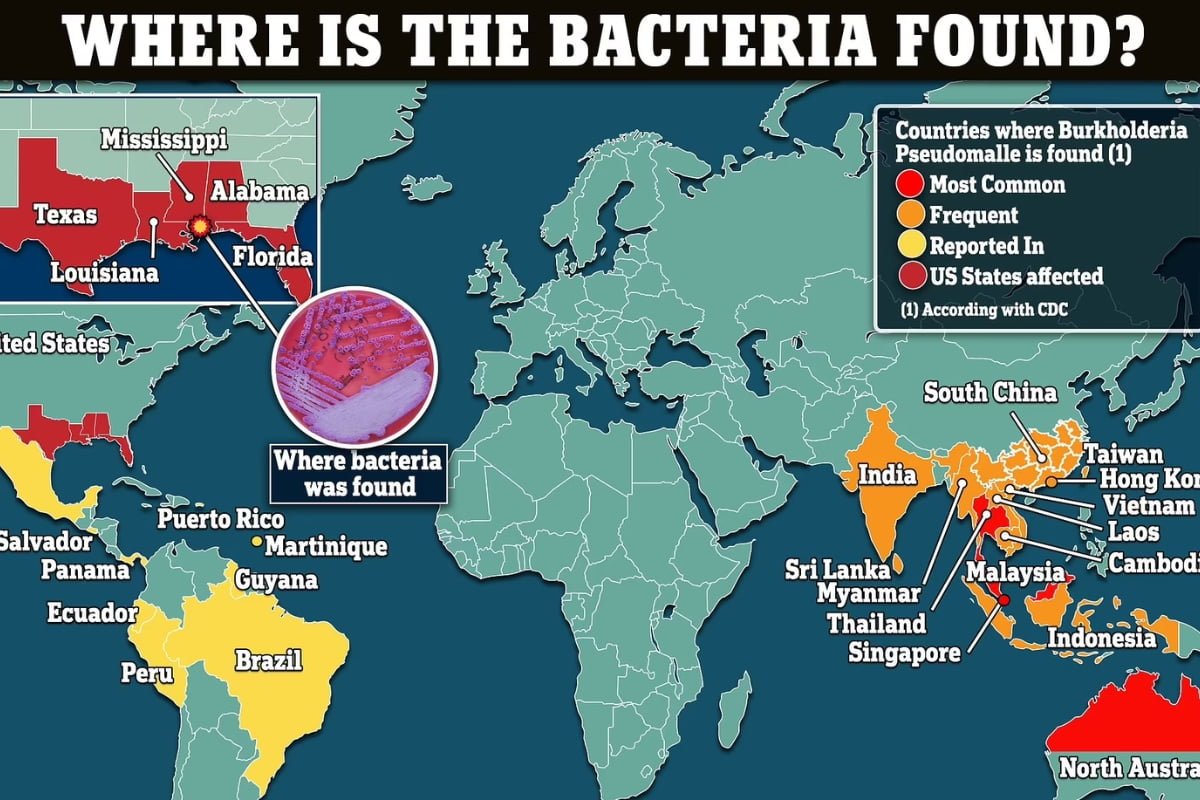
CDC Warns of Deadly Bacteria with 50% Fatality Rate
The alarming announcement made by the CDC regarding a deadly bacteria that has been declared endemic to the US Gulf ...

CDC Raises Concerns about Deadly Bacteria with High Fatality Rate Endemic to US Gulf Coast
A highly dangerous bacterium, known as Burkholderia pseudomallei, has recently been declared endemic to the US Gulf Coast by the ...
Eisai has requested to approve Leqembi, its therapy for Alzheimer’s disease, in South Korea
This filing comes just before an FDA advisory committee meeting, which has raised expectations of the drug receiving full approval ...
FDA accepts Merck’s application for Keytruda plus chemo in liver cancer
The FDA has granted acceptance to Merck’s application for the popular drug Keytruda, in combination with standard-of-care chemotherapy (gemcitabine plus ...
GSK Secures First Approval for RSV Vaccine in EU
The European Commission has given the green light for GSK’s Arexvy to be used in older adults aged 60 or ...
Johnson & Johnson (J&J) Moves Closer to FDA Approval for CAR-T Therapy
The company has filed for approval to use Carvykti earlier in the treatment pathway, including for patients experiencing their first ...
ASCO 2023: Unlocking the Potential of TGF-β Inhibition
ABSTRACT NUMBER: 6026 Recent studies have underscored the crucial role of the TGF-β signalling pathway in shaping the immune microenvironment ...







