Anika Sharma
Phase II DeLLphi-301 Study Reveals Impressive Antitumor Activity of Amgen’s First-in-Class Bispecific Antibody Tarlatamab (ESMO 2023)
ESMO 2023: LBA92 Most SCLC patients are diagnosed with cancer that has already spread beyond the lungs, and their average ...
Promising Outcomes and Safety Profile of Krazati Combined with Pembrolizumab in Treatment-Naïve, Advanced NSCLC Patients with KRASG12C Mutation: Insights from the Phase II/III KRYSTAL-7 Study (ESMO 2023)
ESMO 2023: LBA65 NSCLC with KRAS mutations is a difficult market with few treatment options and many unmet needs. Krazati ...

KEYNOTE-671 Trial: Keytruda Sets New Standards in the Perioperative Treatment Landscape (ESMO 2023)
ESMO 2023: LBA56 Neoadjuvant NSCLC is a challenging market with few treatment options and many unmet needs. Merck’s Keytruda is ...
Keytruda’s Success in KEYNOTE-756 Trial Ushers in a New Era for Early-Stage High-Risk ER+/HER2– Breast Cancer Treatment (ESMO 2023)
ESMO 2023: LBA21 Keytruda (pembrolizumab) is a highly specific, human-made antibody that targets the PD-1 pathway and prevents cancer cells ...
Rybrevant Redefines First-Line Care for Patients with EGFR Exon 20 Insertion Mutation (ESMO 2023)
ESMO 2023: LBA5 Targeted therapies have revolutionized the treatment of EGFR-mutant NSCLC in the last 20 years. However, EGFR exon ...
ESMO 2023: Bristol Myers Squibb’s Opdivo Plus Chemotherapy Improves Survival in Resectable NSCLC: CheckMate -816 Trial
ESMO 2023: Abstract ID 1261O Treating early-stage NSCLC presents numerous obstacles, given the restricted therapeutic alternatives available. In this landscape, ...
How Keytruda Improved Survival Outcomes for Early-Stage TNBC Patients: KEYNOTE-522 Trial
ESMO 2023: LBA18 Underpinning this approval is the seminal KEYNOTE-522 trial, a Phase III study that diverges from pre-2021 tactics ...

ESMO 2023: GSK’s Jemperli beats Merck’s Keytruda in lung cancer survival trial
In a groundbreaking clinical trial, GSK has achieved remarkable patient survival results with its PD-1 inhibitor, Jemperli, when compared to ...
Pfizer’s Penbraya: First vaccine to prevent all five types of meningitis gets FDA approval
Pfizer has secured a significant regulatory victory in the race to introduce the first five-pronged meningitis vaccine in the United ...

Samsung BioLogics faces FDA scrutiny over data integrity and quality issues at Korean plant
The FDA recently issued manufacturing alerts, targeting high-flying companies Samsung Biologics and Nectar Lifesciences. For Samsung Biologics, a notable player ...
Tremfya clears psoriasis in people of color, first study of its kind shows
The journey to improved treatments for psoriasis, a challenging skin condition, has been long and complex. Despite advances, some patient ...

Merck and Daiichi join forces to develop novel cancer drugs in $22B deal
In a strategic move, Merck & Co. has solidified its position in the thriving field of antibody-drug conjugates (ADCs) through ...

Nkarta’s breakthrough cell therapy for lupus gets FDA approval after showing promise in cancer treatment
In a groundbreaking development, Nkarta is pushing the boundaries of cell therapy beyond the realm of cancer. The FDA has ...
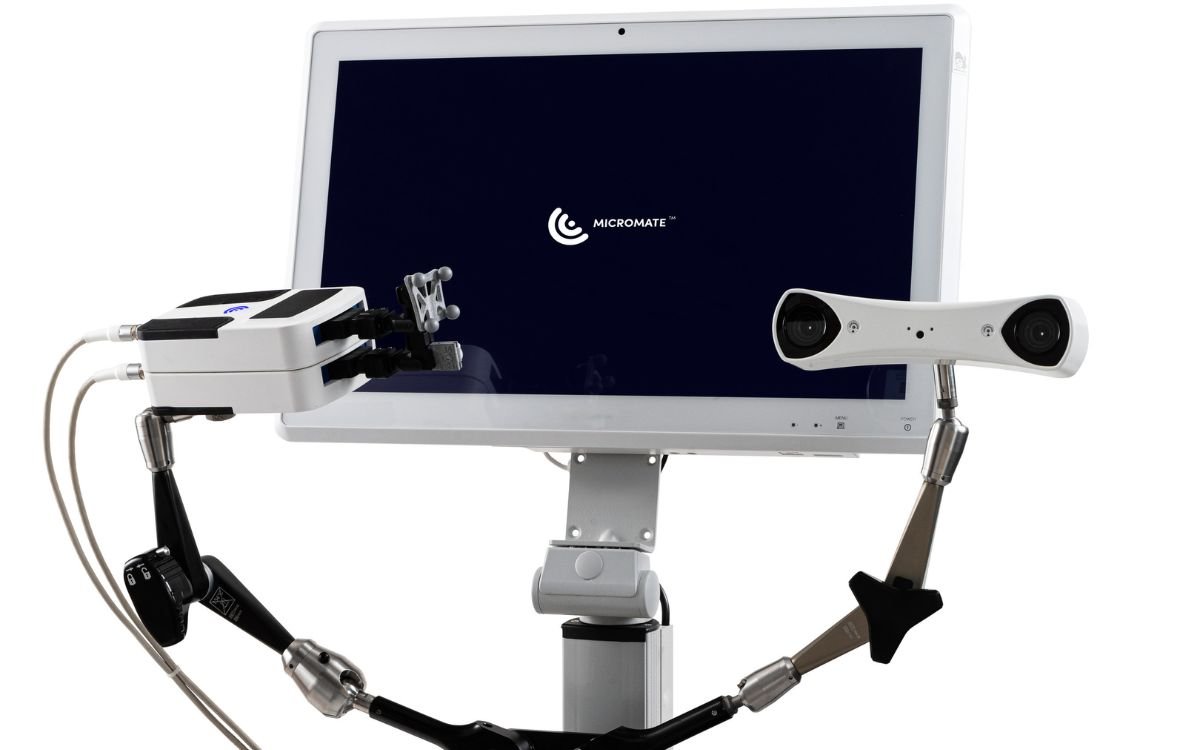
FDA Clearance for Micromate: A tiny robot that can perform needle biopsies with CT imaging
Interventional Systems, the brains behind a compact yet powerful robotic marvel, is set to leave a profound mark in the ...

Pfizer agrees to pay $300,000 to resolve gender pay bias claims at NYC HQ
Pfizer has agreed to a $2 million settlement to resolve allegations made by the US Department of Labor, which claimed ...

BMS and Halozyme announce positive results for subcutaneous Opdivo in cancer patients
Following the discontinuation of an auto-injected version of Opdivo (nivolumab), Bristol Myers Squibb (BMS) has achieved success with a more ...

Fake Ozempic products seized in EU and UK, Novo Nordisk warns
As Novo Nordisk grapples with the challenges posed by illicit sales of semaglutide, authorities in the UK and Europe have ...

Roche unveils its blood cancer strategy after COVID-19 boost
While Roche has seen a decline in COVID-19 drug sales after the pandemic boom, the company is experiencing a resurgence ...

PTC sells royalty rights of SMA drug Evrysdi to Royalty Pharma for $1.5B
As part of its cost-cutting efforts and in response to potential challenges in the European market, PTC Therapeutics is taking ...

Takeda’s Alofisel fails to meet primary endpoint in phase 3 trial for Crohn’s disease complication
Takeda, following its decision to remove an early-stage Crohn’s disease candidate from its pipeline earlier this year, is now contending ...

Avania acquires two medtech CROs, broadens its global services and expertise
Avania, a medtech-focused Contract Research Organization (CRO) headquartered in the Netherlands, has extended its industry expertise through the strategic acquisitions ...

Lilly acquires European biotech Elahere, expands its ADC network and pipeline
Eli Lilly is actively expanding its involvement in the burgeoning field of antibody-drug conjugates (ADCs) in Europe. Following its recent ...

Roche abandons oral drug for eye disease after phase 2 failure, shifts focus from solid tumor bispecifics
Roche’s ambitious quest to develop an oral treatment for diabetic retinopathy has faced a setback, as the company decided to ...

Bimzelx, a potential blockbuster for psoriasis, clears FDA approval after resolving manufacturing issues
UCB’s long-awaited FDA approval for its psoriasis therapy, Bimzelx (bimekizumab), has finally arrived, ending a journey marked by manufacturing setbacks. ...

Basilea buys clinical-stage antifungal from Eisai, advances its anti-infective strategy
Basilea Pharmaceutica is making a strategic move to bolster its anti-infective pipeline, this time with a $2 million investment in ...

Zilbrysq, A Complement C5 Inhibitor For Myasthenia Gravis, Gets FDA Approval As UCB’s Second Drug For The Rare Disease
UCB is celebrating an impressive streak of FDA approvals, securing the green light for both the plaque psoriasis treatment Bimzelx ...

Johnson & Johnson Overcomes CAR-T Drug Carvykti’s Launch Challenges with Manufacturing Improvements
Johnson & Johnson (J&J) and Legend Biotech are determined to expand the reach of their CAR-T drug Carvykti for multiple ...