Anika Sharma

FDA Convenes Advisors to Assess Full Approval Prospects for Amgen’s Lumakras Amid KRAS Uncertainties
Amgen faces a critical juncture with its pioneering KRAS inhibitor, Lumakras, as uncertainties loom over its potential combinability with standard ...

Agilent Pulls the Plug on $695M Resolution Cancer Companion Diagnostic Venture, 2 Years After Acquisition
Following its acquisition of Resolution Bioscience for a sum exceeding $500 million, Agilent Technologies has made the decision to close ...

Fulcrum’s Sickle Cell Disease Program Resumes as FDA Lifts 6-Month Hold
Fulcrum Therapeutics’ ambitions in the realm of sickle cell disease (SCD) have been revitalised after a six-month hiatus. The FDA ...

Aravive’s Desperate Bid for Survival: Drastic Layoffs as Company Nears Liquidation
Aravive, a biotech company, is currently facing a critical juncture as it grapples with the aftermath of a disappointing Phase ...

Ultimovacs Explores Innovative Approaches to Conclude Phase 2 Cancer Trial Amid Prolonged Anticipation for Survival Data
Ultimovacs, a Norwegian biotech company, is grappling with prolonged delays in obtaining midphase melanoma trial data, prompting the company to ...

ICU Medical to layoff 82 workers, wind down operations at Minnesota facility
In a strategic move, ICU Medical has revealed plans to permanently shut down a manufacturing facility in Minnesota, a decision ...
Hutchmed Sets Sights on Chinese Approval for Autoimmune Disorder Drug Following Phase 3 Triumph
Hutchmed, a prominent pharmaceutical player, is gearing up for a significant move as it readies to submit sovleplenib for regulatory ...
CARsgen and Moderna Join Forces to Explore Synergy Between CT041 and mRNA Cancer Vaccine
CARsgen Therapeutics has set the stage for an intriguing partnership with Moderna that’s bound to heat up the cancer therapy ...

Gilead’s Magrolimab Faces Fresh Setback as FDA Issues Partial Clinical Hold
One month after Gilead Sciences made public its decision to halt a myelodysplastic syndromes (MDS) program centered around magrolimab, the ...

LEO Pharma Set to Acquire Timber Pharmaceuticals
Timber Pharmaceuticals has exciting news to share—the company is set to be acquired by LEO US Holding, Inc., a wholly-owned ...
AARP Backs Justice Department Against Chamber of Commerce’s Lawsuit on Medicare Drug Price Talks
A new development has emerged in the legal battle over the Inflation Reduction Act’s (IRA) provisions on prescription drug prices. ...
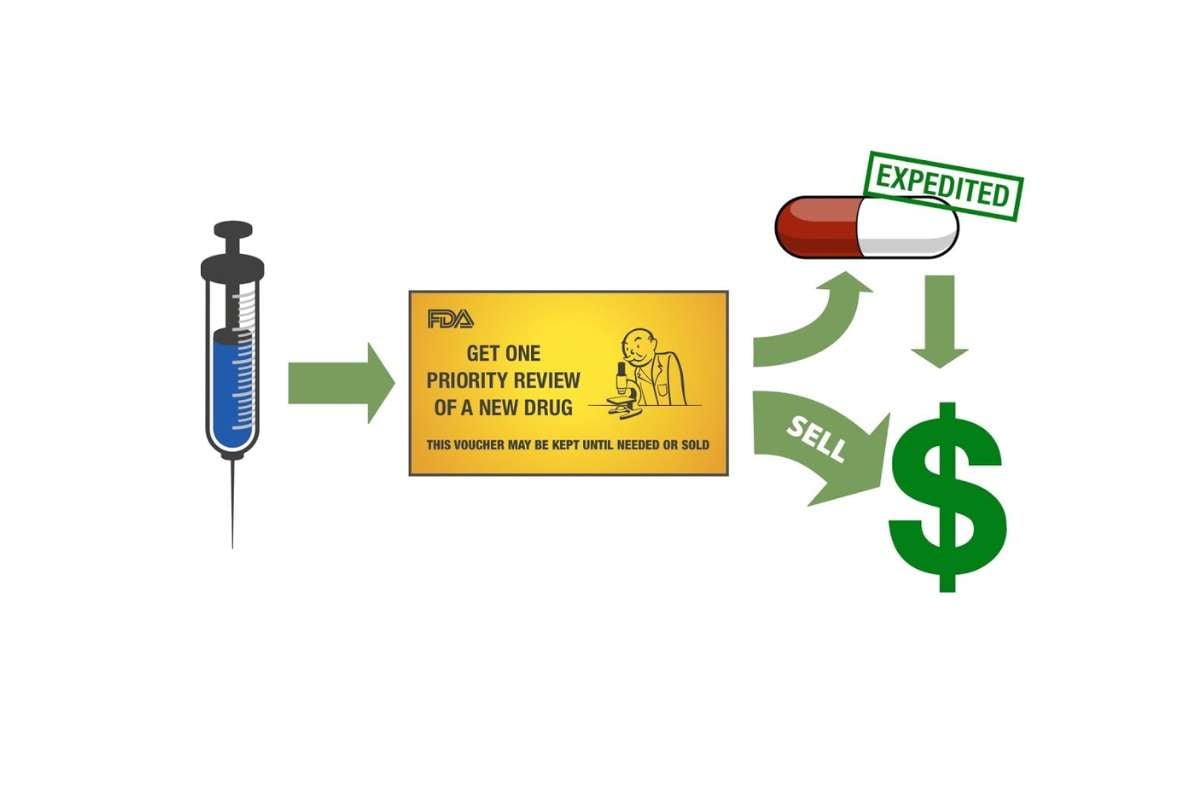
Krystal Biotech Secures $100 Million in Sale of Priority Review Voucher
Krystal Biotech proudly announces the successful transaction of its Rare Pediatric Disease Priority Review Voucher (PRV), yielding an impressive $100 ...

Johnson & Johnson’s Strategic Move: Retains 9.5% Stake in Kenvue After Share-Exchange Offer
Following its share-exchange initiative aimed at reducing its stake in Kenvue, Johnson & Johnson has announced that it will maintain ...
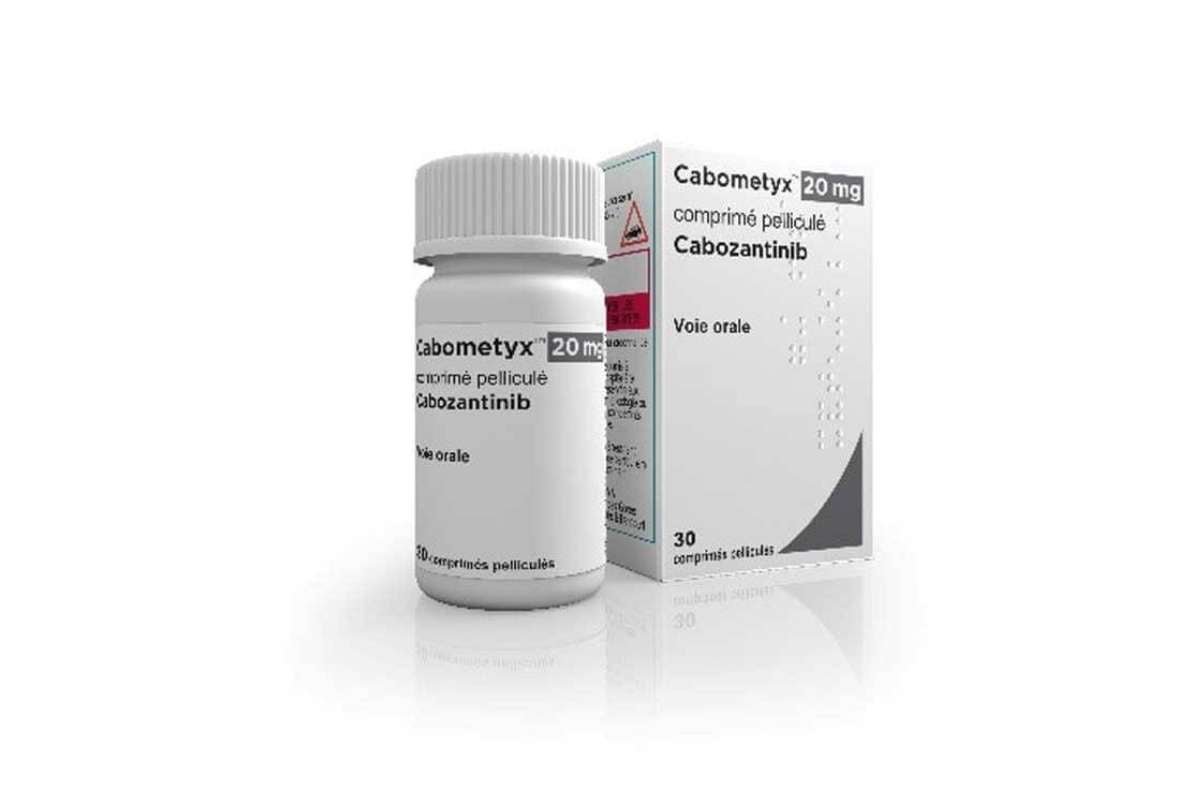
Resilient Triumph: Roche and Exelixis’ Tecentriq-Cabometyx Combination Shows Promise in Advanced Prostate Cancer After Past Challenges
After facing setbacks in clinical trials, the combination of Roche’s Tecentriq and Exelixis’ Cabometyx has achieved a breakthrough in treating ...
Phathom Pharmaceuticals Boosts Erosive GERD Application with Six-Month Stability Data Submission for Vonoprazan
Phathom Pharmaceuticals, a pioneering biopharmaceutical company dedicated to groundbreaking solutions for gastrointestinal ailments, has announced a significant stride in its ...
CAR T-Cell Therapy in Hematological Malignancies: A Breakthrough or a Bust?
Hematological malignancies are cancers that affect the blood, bone marrow, and lymph nodes. They include leukemia, lymphoma, and multiple myeloma. ...
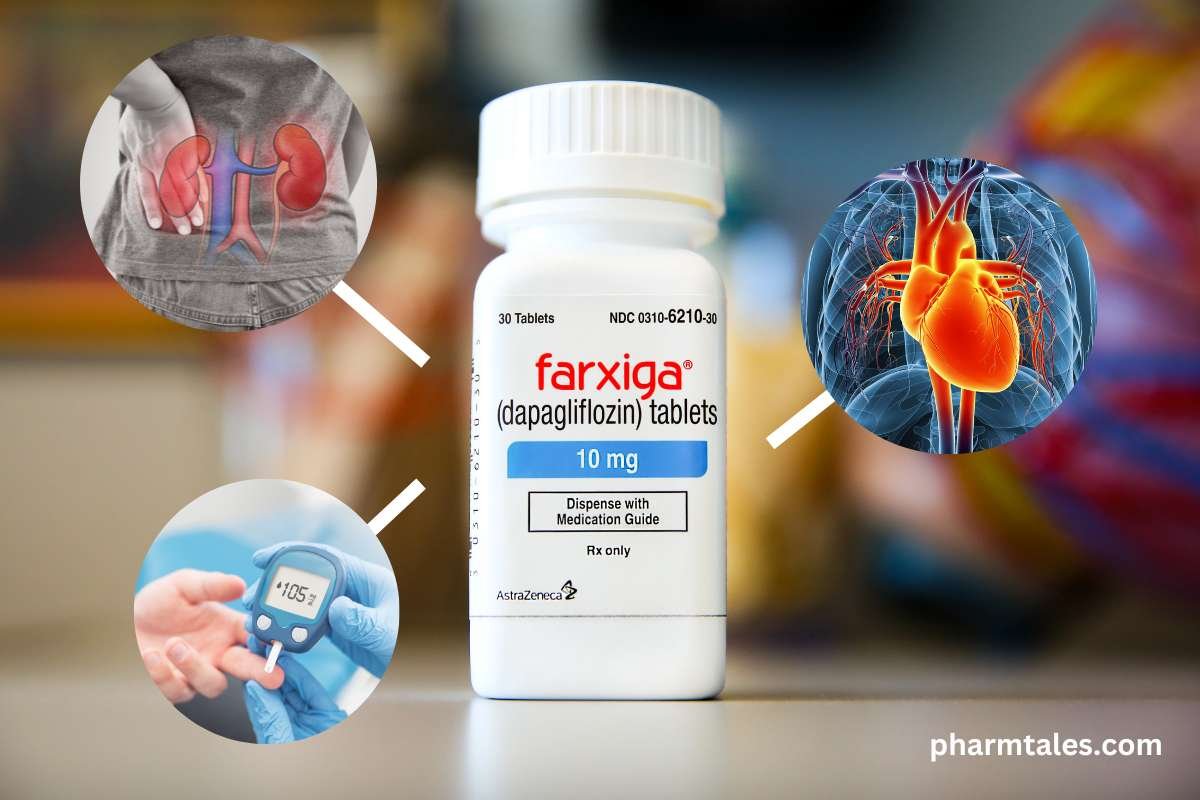
Farxiga/Forxiga: Drug for Diabetes, Heart Failure and Kidney Disease
Farxiga is a brand name in the United States and Forxiga is the brand name in Europe and other countries. ...

FDA Awards Priority Review for Full Approval of Tarpeyo in Treating IgA Nephropathy
Calliditas Therapeutics, a leading pharmaceutical company, has exciting news to share. The U.S. Food and Drug Administration (FDA) has accepted ...
FDA Approves Ingrezza (valbenazine) Capsules to Treat Chorea in Huntington’s Disease
Neurocrine Biosciences has achieved a significant milestone with the announcement of US Food and Drug Administration (FDA) approval for Ingrezza ...

Janssen’s Bispecific Antibody Tecvayli Receives European Commission Approval for Reduced Dosing Frequency
The Janssen Pharmaceutical Companies of Johnson & Johnson have proudly unveiled a significant advancement in cancer treatment. The European Commission ...

FDA Grants Landmark Approval to Veopoz for the Treatment of Pediatric and Adult CHAPLE Disease
On August 18, 2023, Regeneron Pharmaceuticals announced a remarkable milestone. The US Food and Drug Administration (FDA) has granted approval ...

Diversifying Strategies as Vaccine Demand Shifts: COVID-19 Vaccine Manufacturers Anticipate Fall Inoculation Season
Amidst a challenging environment of declining demand for COVID-19 vaccines, producers are eagerly awaiting the upcoming fall inoculation season as ...
Cancer-Linked HPV Carried by One in Five Men: Study Reveals Startling Prevalence
New research findings have shed light on the widespread prevalence of genital human papillomavirus (HPV) infections among men globally. A ...

Novartis Nears Sandoz Spinoff Milestone with Unique Stock Distribution Strategy
As the long-anticipated spinoff of Novartis’ Sandoz division draws near, the Swiss pharmaceutical giant has unveiled a novel approach to ...

Merck Sets Sights on Transforming Welireg into Blockbuster Drug, Expanding Treatment Scope for RCC Patients
In a strategic move to widen the impact of Welireg, Merck is poised to extend the drug’s influence and elevate ...

AI Therapeutics Launches Phase 2 Trial of LAM-001 for Bronchiolitis Obliterans Syndrome Post-Lung Transplant
AI Therapeutics, a forward-looking clinical-stage biopharmaceutical company dedicated to pioneering novel therapies for rare diseases, has announced the initiation of ...

Forxiga Receives Green Light in China to Mitigate Cardiovascular Mortality and Hospitalization in Adults Battling Symptomatic Chronic Heart Failure
China’s National Medical Products Administration (NMPA) has granted approval to Forxiga (dapagliflozin) as a groundbreaking solution in the fight against ...