Committee for Medicinal Products for Human Use
PTC cuts jobs as Europe withdraws approval for its DMD drug
PTC Therapeutics has announced an expansion of its previously planned workforce reductions as European regulatory concerns cast a shadow over ...

PTC Therapeutics’ Translarna at risk of losing EU approval after failing to impress FDA for DMD
PTC Therapeutics is facing a significant setback as it seeks to maintain approval for its Duchenne muscular dystrophy (DMD) therapy, ...
Pfizer and BioNTech Get Green Light for Omicron Vaccine in Europe by CHMP
Pfizer and BioNTech have unveiled their latest breakthrough in the post COVID era. Brace yourselves for the Omicron XBB.1.5-adapted monovalent ...
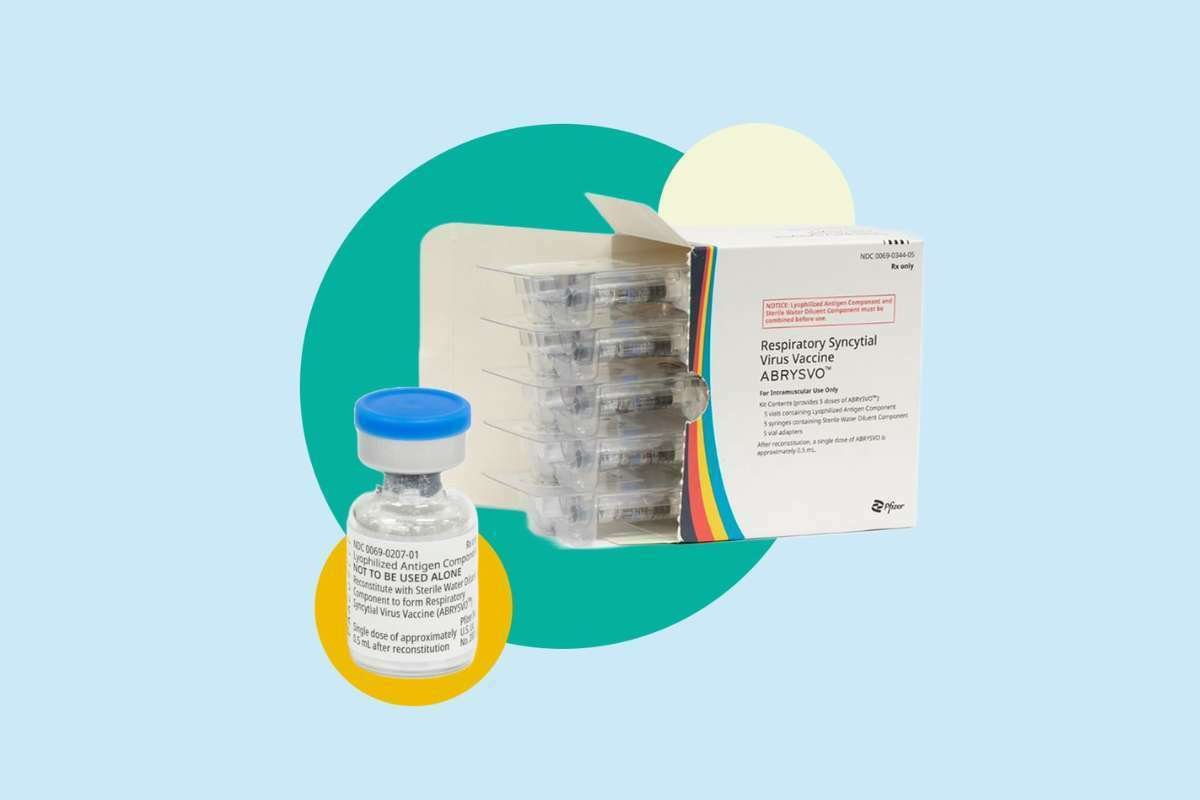
EC Approves Pfizer’s Abrysvo, Only RSV vaccine in EU for adults and infants
Pfizer has just received approval from the European Commission (EC) for its groundbreaking vaccine, Abrysvo. This vaccine, targeting the respiratory ...
AbbVie Receives Favorable CHMP Opinion for Atogepant as Preventive Treatment for Migraine in Adults
Source – AbbVie AbbVie announced on June 23, 2023, that the Committee for Medicinal Products for Human Use (CHMP) of ...








