CHMP Opinion

EU regulator denies approval for Amylyx’s Albrioza, a potential ALS treatment
While Amylyx’s ALS drug, known as Albrioza in Europe and Relyvrio in the US, has made significant strides in the ...
Enhertu, a Breakthrough Therapy for HER2 Mutant NSCLC, Gets EU Nod
Enhertu (trastuzumab deruxtecan) has received a positive recommendation for approval in the European Union (EU) as a standalone treatment for ...
Quizartinib Gets Green Light in EU for Treating Acute Myeloid Leukemia
Daiichi Sankyo has received a positive recommendation for the approval of quizartinib in the European Union (EU). This recommendation is ...
A New Hope for Children and Teens with gMG: Soliris Gets EU Approval
Source – AstraZeneca The European Union (EU) has granted approval for the expanded use of Soliris (eculizumab) to treat refractory ...

Merck’s Gefapixant Receives Positive EU CHMP Opinion
Source – Merck MSD has announced that the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has ...

AbbVie’s Epcoritamab (EPKINLY) Receives Positive CHMP Opinion for DLBCL Treatment
Source – AbbVieAbbVie and Genmab’s potential blockbuster drug epcoritamab has received a favorable recommendation for conditional approval in the EU ...
Sandoz’s Biosimilar Natalizumab Receives Positive CHMP Opinion for Multiple Sclerosis Treatment
Source – Novartis On July 24, 2023 Sandoz announced a significant milestone. The Committee for Medicinal Products for Human Use ...

EU CHMP Grants Favorable Opinion to Merck’s Keytruda Combination for HER2-Positive Advanced Gastric Cancer
Source – Merck A new hope for patients with advanced gastric or GEJ cancer that is positive for both HER2 ...
CHMP Backs Approval of Pembrolizumab Combination Therapy for HER2+ Gastric Cancer in the EU
Source – Merck A new combination therapy for patients with advanced gastric or GEJ cancer that is positive for both ...
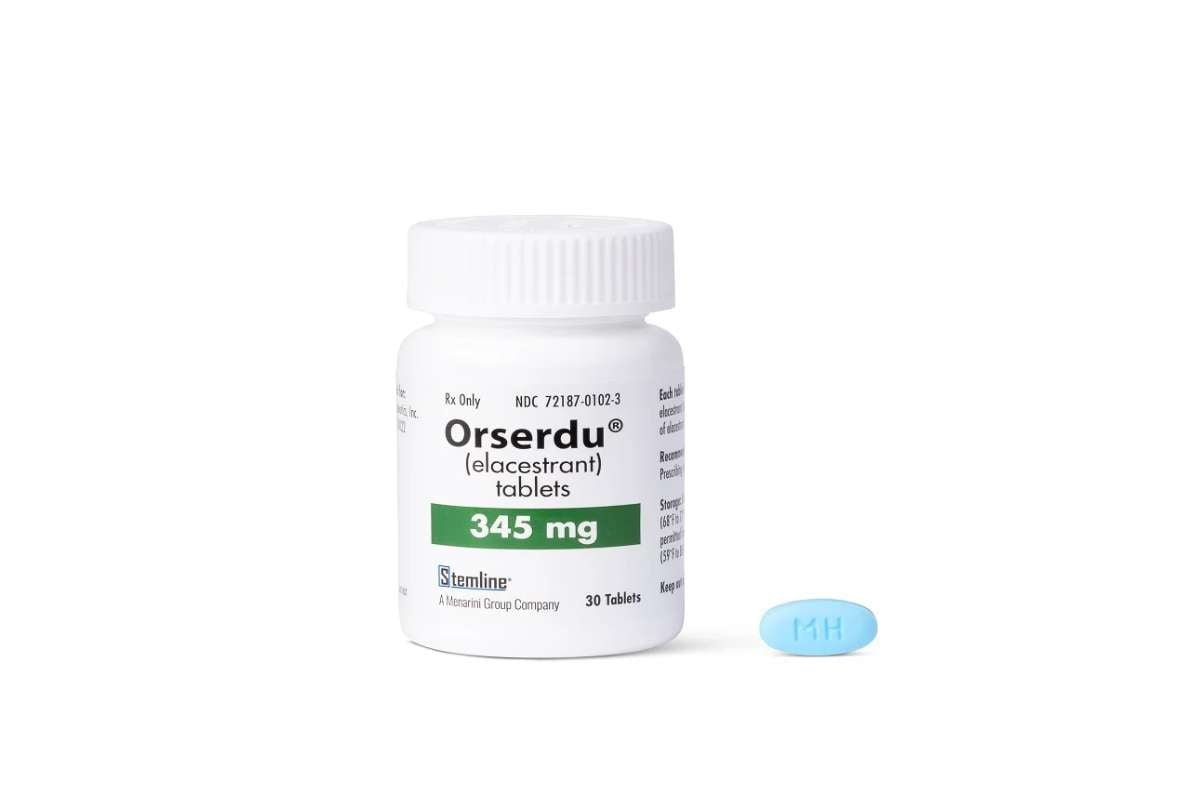
CHMP Greenlights Elacestrant for Advanced Breast Cancer Treatment in the EU
Source – Menarini Group A new treatment option for postmenopausal women with advanced or metastatic breast cancer has moved a ...
Regulatory Update: Amylyx’s ALS drug faces rejection by the European Medicines Agency (EMA)
Source – Amylyx Pharmaceuticals On June 23, 2023, the European Medicines Agency (EMA) has formally issued a negative opinion regarding ...

Opdivo Receives Positive CHMP Opinion for Neoadjuvant Treatment of High-Risk NSCLC
On May 26, 2023, Bristol Myers Squibb announced that the European Medicines Agency’s Committee for Medicinal Products for Human Use ...







