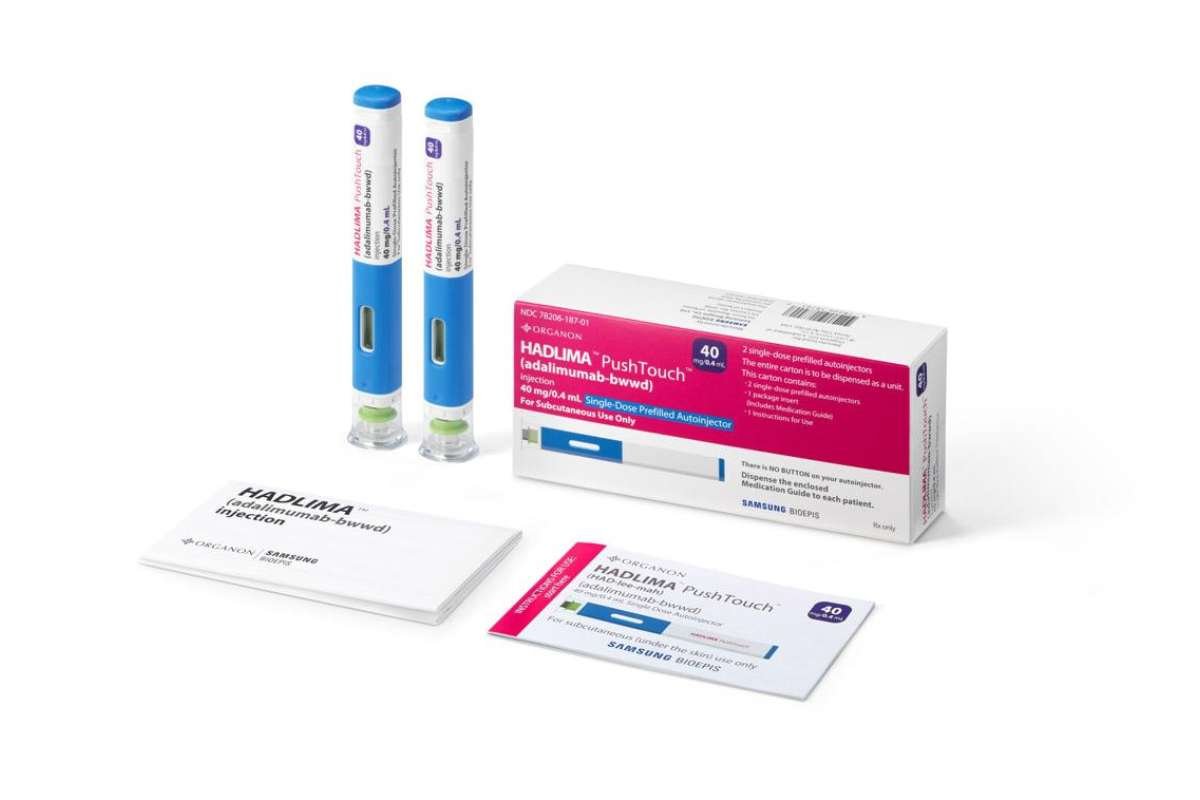
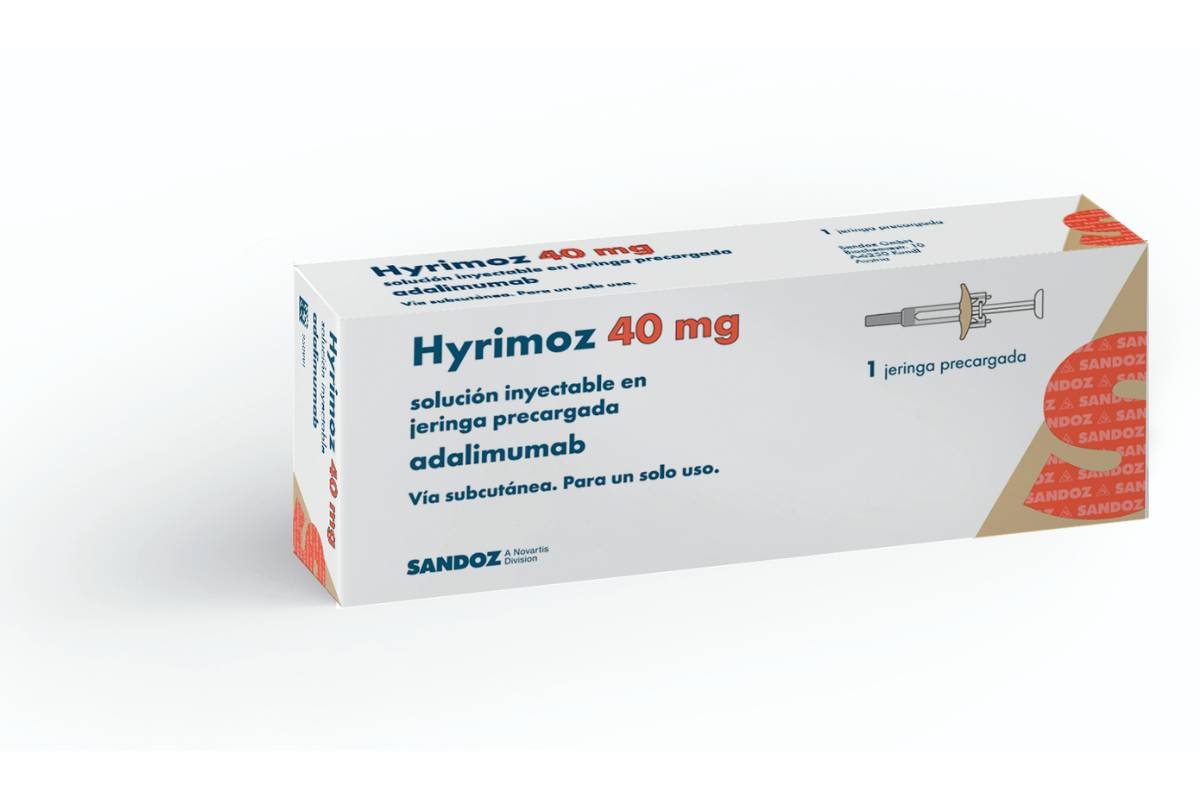
Humira Biosimilars: Benefits, Availability, Price, Comparison and More
In this article, we will give you an overview of Humira biosimilars, including their benefits, availability, prices, and how they compare to Humira. We will also answer some frequently asked questions about Humira biosimilars and provide some tips on how to switch from Humira to a biosimilar safely and smoothly. Humira is a brand-name medication … Read more