Source: Pfizer
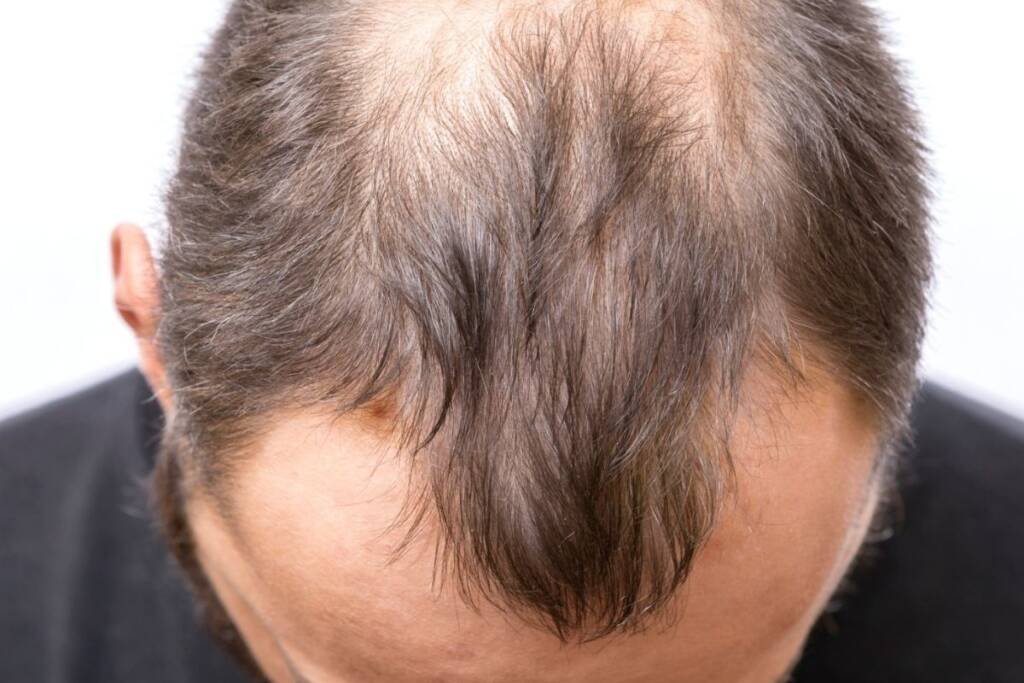
Pfizer announced on June 23, 2023, that the U.S. Food and Drug Administration (FDA) has granted approval to LITFULO (ritlecitinib), an oral treatment taken once daily, for severe alopecia areata in individuals aged 12 and above. LITFULO is the first and only FDA-approved treatment for severe alopecia areata in adolescents.
LITFULO acts as a kinase inhibitor, specifically targeting Janus kinase 3 (JAK3) and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) family of kinases.
“While patients may start to develop symptoms of alopecia areata at any age, most people start showing signs in their teens, twenties, or thirties. LITFULO is a particularly important treatment option for younger patients with substantial hair loss, who often struggle with such a visible disease.”
– Dr. Brittany Craiglow, Associate Professor Adjunct – Dermatology at Yale School of Medicine
The FDA approval was based on clinical trial results focusing on alopecia areata. The pivotal ALLEGRO Phase IIb/III trial enrolled 718 patients with 50% or more scalp hair loss, as measured by the Severity of Alopecia Tool (SALT), across 118 sites in 18 countries. The trial evaluated the efficacy and safety of LITFULO. After six months of treatment, 23% of patients receiving LITFULO 50 mg achieved 80% or more scalp hair coverage (SALT≤20), compared to 1.6% of those on placebo. The efficacy and safety of LITFULO were consistent across both adolescents (12 to 17 years of age) and adults (18 years of age and older). Common adverse events (AEs) reported by at least 4% of patients treated with LITFULO included headache (10.8%), diarrhea (10%), acne (6.2%), rash (5.4%), and urticaria (4.6%). The complete findings of the ALLEGRO Phase IIb/III study were published in April 2023 in The Lancet.
“LITFULO is an important treatment advancement for alopecia areata, an autoimmune disease that previously had no FDA-approved options for adolescents and limited options available for adults. With today’s approval, adolescents and adults who struggle with substantial hair loss have an opportunity to achieve significant scalp hair regrowth.”
– Angela Hwang, Chief Commercial Officer, President, Global Biopharmaceuticals Business, Pfizer