Approvals
Stay up-to-date with the latest drug approvals by regulatory agencies worldwide. Gain insights into the regulatory processes, safety evaluations, and efficacy assessments. Stay informed about the new treatments available to patients and the impact they have on healthcare practices.

The Arrival of OTC birth control in the US
Source – FDA The US has approved the first-ever over-the-counter (OTC) contraceptive pill, providing women with a new option for ...

The first respiratory syncytial virus (RSV) vaccine for elderly people, GSK’s Arexvy, received approval from the Medicines and Healthcare Products Regulatory Agency
Source – GSK GSK has announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK has granted ...

Better receives FDA approval for its primary DTx medication
Source –Better Therapeutics On 10 July, Digital health company Better Therapeutics received FDA approval for its lead digital therapeutic (DTx), ...

Roche’s Columvi (glofitamab) for patients with R/R diffuse large B-cell lymphoma has received approval from the European Commission
Source – Roche On 11 July 2023 Roche announced that the European Commission (EC) has given conditional approval for Columvi ...

Novavax’s Covid vaccine receives a unanimous EU approval
Source – Novavax Novavax received full Marketing Authorization (MA) from the European Commission for its COVID-19 vaccine, Nuvaxovid (NVX-CoV2373). This ...
European Commission Grants Approval for ORKAMBI to Treat Cystic Fibrosis in Children Aged 1 to <2 Years
Source – Vertex Pharmaceuticals On July 5, 2023, Vertex Pharmaceuticals announced that the European Commission has granted approval for the ...

FDA Grants Approval to Epcoritamab– Redefining the Future of DLBCL
In the world of cancer treatment, groundbreaking advancements are constantly being made, offering new hope for patients and their loved ...

Eisai and Biogen Embark on Full Launch of Leqembi Following Alzheimer’s Approval
Source – Eisai On July 6, 2023, Eisai and Biogen received full approval from the FDA for their Alzheimer’s disease ...
Roche withdraws Gavreto approval, citing study impracticality
Roche’s cancer drug Gavreto was withdrawn for the treatment of advanced RET-mutant medullary thyroid cancer, following discussions with the FDA. ...
FDA Approves Cyclophosphamide Injection NDA for Multiple Cancers
Source – Nevakar Injectables On July 3, 2023 the FDA has approved a new drug application (NDA) for 200-mg/mL vials ...
First cell treatment for type I diabetes is approved by the FDA
Source – FDA On June 29, 2023, CellTrans achieved a significant milestone by securing the first-ever FDA approval for a ...
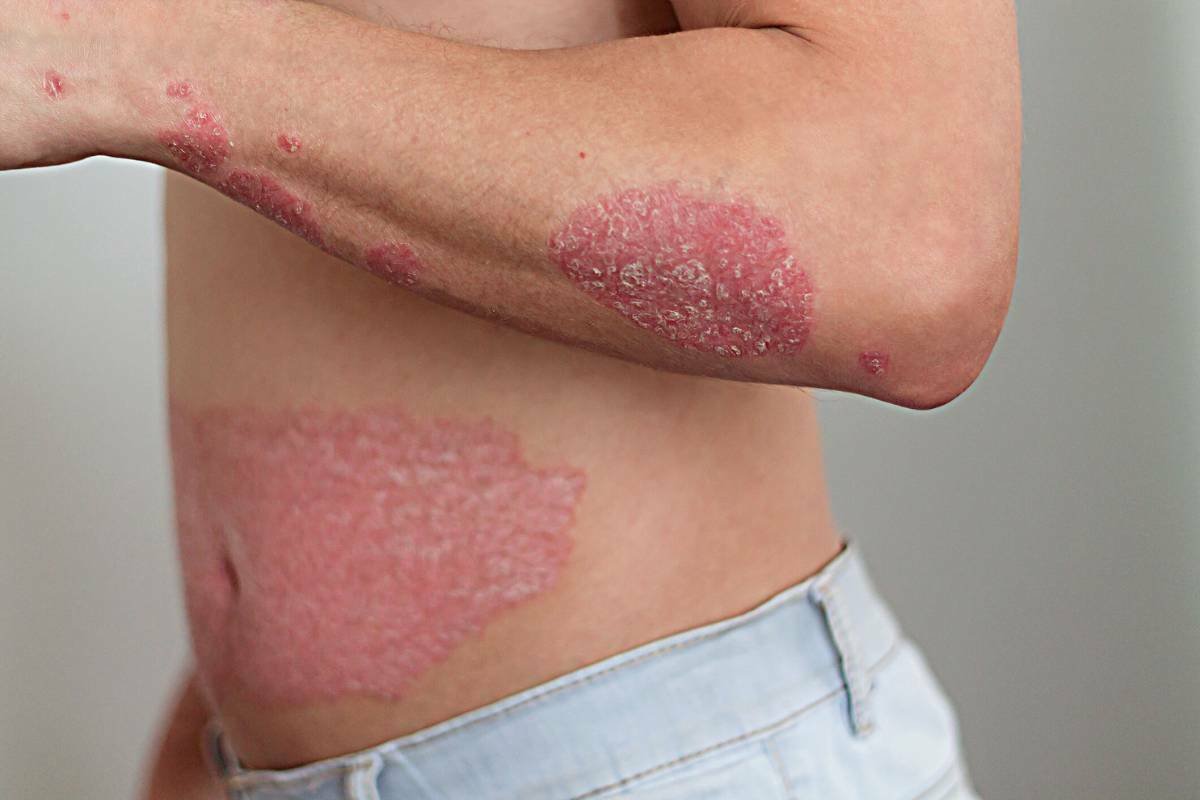
BMS’ Sotyktu is approved for psoriasis usage by the NHS
Source: Bristol-Myers Squibb Just weeks after receiving approval in the UK, Bristol-Myers Squibb’s novel oral therapy for psoriasis, SotyKtu, has ...
BioMarin’s Roctavian: FDA Approves Hemophilia A Gene Therapy with Positive Reception from Physician
Source – BioMarin BioMarin’s Roctavian, a gene therapy for severe hemophilia A, has received FDA approval after initial rejection in ...
Shingrix Granted Approval by Japan’s Ministry of Health Labour and Welfare for Shingles Prevention in At-Risk Individuals 18 Years and Older
Source – GSK On 26 June 2023, GSK announced that the Japanese Ministry of Health, Labour and Welfare (MHLW) has ...
Reata Pharmaceuticals Begins Launching Its First Commercial Product Skyclarys with Approved Supplement
Source – Reata Pharmaceuticals After receiving approval in the spring, Reata Pharmaceuticals has been eagerly awaiting the launch of its ...

Pediatric Growth Hormone Deficiency Long-Acting Once-Weekly Treatment NGENLA by Pfizer Receives FDA Approval
Source – Pfizer On 29 June 2023, Pfizer and OPKO Health jointly announced that the US Food and Drug Administration ...
UCB has achieved a long-awaited victory as the FDA grants approval for the myasthenia gravis drug Rystiggo
Source – UCB On 27 June 2023, UCB faced regulatory challenges in the United States for the second consecutive year, ...
Xigduo XR Receives Approval in China for the Treatment of Type II Diabetes in Adults
Source – AstraZeneca On June 27, 2023, AstraZeneca’s Xigduo XR, a once-daily fixed-dose combination of dapagliflozin and metformin hydrochloride extended-release, ...
FDA Grants Approval to Pfizer’s LITFULO (Ritlecitinib) for Severe Alopecia Areata in Adults and Adolescents
Source: Pfizer Pfizer announced on June 23, 2023, that the U.S. Food and Drug Administration (FDA) has granted approval to ...
Talazoparib Plus Enzalutamide for HRR Gene-Altered mCRPC Receives FDA Approval
Source – FDA On June 20, 2023, the combination of talazoparib (Talzenna) and enzalutamide (Xtandi) has been approved by the ...

Argenx receives FDA approval for the first gMG subcutaneous treatment
Source – Argenx Argenx has claimed an FDA clearance that might increase its market share in the increasingly competitive market ...

The US FDA has approved the use of Jardiance (empagliflozin) to treat type II diabetes in children and adolescents aged 10 and older
Source – Eli Lilly On June 21, 2023, Boehringer Ingelheim and Eli Lilly and Company announced that the US Food ...

Glofitamab-gxbm receives FDA approval for relapsing or resistant DLBCL
Source – Roche Columvi (Glofitamab-gxbm) has been given expedited clearance by the FDA for the treatment of adult patients with ...

Companion Diagnostic for Encorafenib + Cetuximab in BRAF V600E alteration in mCRC Receives FDA Approval
Source – Foundation Medicine In order to identify patients with metastatic colorectal cancer (mCRC) who could be candidates to receive ...

FDA authorizes the first medication for functional constipation in children
Source- FDA Pediatric patients aged 6 to 17 may use Linzess (linaclotide) pills to treat functional constipation. The first therapy ...

Futura receives FDA approval for OTC treatment for erectile dysfunction
Source- Futura Medical The first business in the US to provide a topical erectile dysfunction medication to the market without ...

For the treatment of people with refractory generalized myasthenia gravis, Soliris has received approval in China
Source- Astrazeneca For the treatment of adult patients with refractory generalized myasthenia gravis (gMG) and anti-acetylcholine receptor (AChR) antibody positivity, ...
FDA Advisory Committee Unanimously Recommends Nirsevimab for Preventing RSV Lower Respiratory Tract Disease in Infants
Source: AstraZeneca The FDA’s Antimicrobial Drugs Advisory Committee (AMDAC) has voted unanimously, with a 21 to 0 decision, affirming the ...

European Commission Approves COSENTYX as First and Only IL-17A Inhibitor for Hidradenitis Suppurativa
Source: Novartis On June 1, 2023, Novartis announced that the European Commission (EC) has granted approval for the use of ...

FDA Advisory Committee Vote Validates LEQEMBI Alzheimer’s Treatment
Source: Eisai On June 9, 2023, the Peripheral and Central Nervous System Drugs Advisory Committee (PCNS) of the US Food ...







