Bristol Myers Squibb (BMS) is making significant strides in the field of pulmonary fibrosis treatment, particularly with its lysophosphatidic acid receptor 1 (LPA1) antagonist, BMS-986278. After demonstrating positive results in a more common form of pulmonary fibrosis in May, this experimental drug has now achieved success in a rarer variation of the disease, known as progressive pulmonary fibrosis (PPF). The findings, presented at the European Respiratory Society 2023 international congress, suggest a promising future for BMS-986278 in the treatment of interstitial lung diseases.
In a phase 2 study, patients with PPF who received two daily 60-mg doses of BMS-986278 experienced a remarkable 69% reduction in the rate of decline in percent predicted forced vital capacity (ppFVC), a critical measure of lung function. Importantly, nearly 38% of the participants were already undergoing treatment with antifibrotic therapies like Roche’s pirfenidone (Esbriet) or Boehringer’s nintedanib (Ofev). BMS-986278’s success, even in combination with existing treatments, suggests its potential as an adjunct therapy.
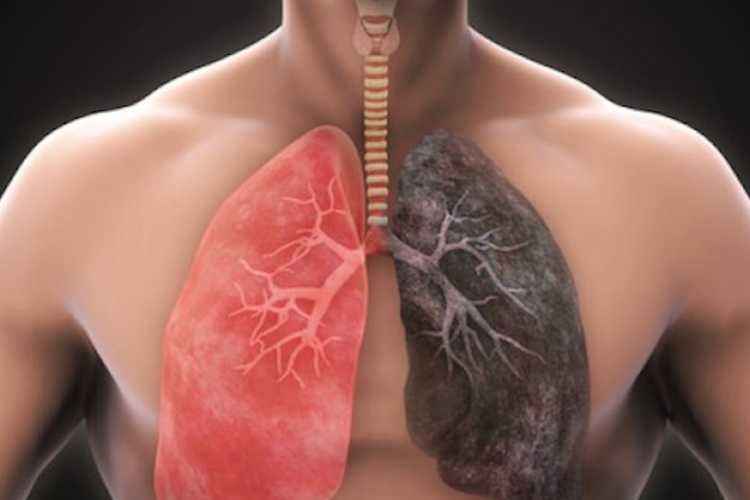
Pulmonary fibrosis is characterized by lung tissue damage and scarring, leading to symptoms such as shortness of breath, coughing, and fatigue. PPF, although less common than its counterpart idiopathic pulmonary fibrosis (IPF), shares a similar grim prognosis, with a median survival time of three to five years and a five-year survival rate of approximately 45%.
“People living with pulmonary fibrosis are in urgent need of new treatment options for this devastating disease, which has a median overall survival of 3-5 years. The Phase 2 progressive pulmonary fibrosis results, which demonstrate consistent efficacy with or without background antifibrotic therapy and a favorable tolerability profile, reinforce the potential of BMS-986278 and highlight advancements in the space as we race to find a potential new standard of care.”
– Professor Tamera J. Corte, clinical trial investigator and Consultant Respiratory Physician and Director of Interstitial Lung Disease, Department of Respiratory Medicine, Royal Prince Alfred Hospital
BMS conducted parallel cohorts for both IPF and PPF patients, administering either 30 mg or 60 mg of BMS-986278 twice daily over 26 weeks. The lower dose of the drug achieved a 42% relative reduction in forced vital capacity compared to the placebo, indicating the importance of dosage in achieving efficacy.
Despite the different doses, BMS noted that treatment results remained consistent, whether patients were on background antifibrotics like Esbriet and Ofev or not. Side effect rates were comparable to the placebo, and discontinuation rates were low. Common side effects included diarrhea, COVID-19 (presumably reported due to the ongoing pandemic), cough, and difficulty breathing.
This success with PPF follows positive phase 2 results for BMS-986278 in IPF, where the 60-mg dose reduced the rate of decline in ppFVC by 62%. Given these promising outcomes, BMS is now gearing up for a phase 3 program, known as the ALOFT (An LPA1 antagonist for Pulmonary Fibrosis Trial) program.
“The results from this innovative study investigating idiopathic and progressive pulmonary fibrosis give us unprecedented insights that will inform our scientific understanding of pulmonary fibrosis and the role of LPA1 inhibition. Our industry-leading drug discovery and development capabilities and collective results from this Phase 2 study provide us the expertise and confidence to support continued development of BMS-986278 in our global Phase 3 ALOFT program in idiopathic and progressive pulmonary fibrosis.”
– Jonathan Sadeh, MD, MSc, senior vice president of Immunology Development, Bristol Myers Squibb
This development places increased pressure on established interstitial lung disease treatments, including Roche’s Esbriet and Boehringer’s Ofev, both of which have enjoyed blockbuster sales over the years. In 2022, Ofev recorded full-year sales of 3.2 billion euros (about $3.4 billion), while Esbriet generated full-year 2022 sales of 718 million Swiss francs (approximately $805 million). It’s noteworthy that Ofev retains patent protection until 2029.
BMS is now at the forefront of advancing novel treatments for pulmonary fibrosis, offering new hope to patients suffering from this debilitating condition