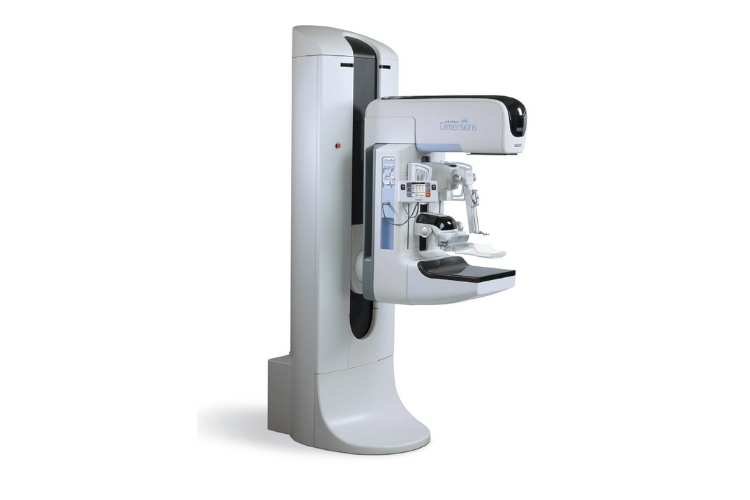
Bayer and Hologic have joined forces with the aim of providing enhanced breast cancer scans by combining Bayer’s imaging contrast agents with Hologic’s mammography hardware. This collaboration is centered on the emerging field of contrast-enhanced mammography (CEM), which complements traditional scans by using contrast agents to highlight blood flow and visualise vessels associated with tumours.
CEM serves as a valuable option when previous breast scans yield inconclusive results or during the pre-surgery planning phase. It can also offer a cost-effective alternative to contrast-enhanced MRI, particularly when MRI machines are unavailable or unsuitable for a patient’s condition, according to Bayer and Hologic.
The partnership’s primary focus will be on Europe, Canada, and the Asia-Pacific region. The companies plan to provide hands-on training to radiologists and clinicians regarding the administration of injectable contrast agents during digital mammography procedures.
“Over the past several years, we’ve seen an increased interest in contrast-enhanced mammography as an additional diagnostic modality. Our partnership with Bayer will enable clinicians around the world to offer CEM as part of the breast cancer diagnostic workflow.”
– Tanja Brycker, Hologic’s VP of strategic development for breast and skeletal health
In January of this year, Bayer obtained European approval for its Ultravist contrast agent for use in CEM. Ultravist, an iodine-based agent, had previously been employed in X-rays, angiography, and CT scans and has been utilized in over 100 countries for various types of scans, including those of the head, chest, heart, abdomen, and liver. Furthermore, the FDA granted CEM approval for this agent in June.